A direct role for GRASP65 as a mitotically regulated Golgi stacking factor
- PMID: 12839990
- PMCID: PMC165642
- DOI: 10.1093/emboj/cdg317
A direct role for GRASP65 as a mitotically regulated Golgi stacking factor
Erratum in
- EMBO J. 2003 Aug 1;22(15):4026
Abstract
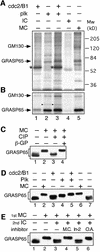
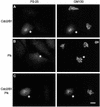
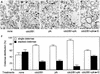
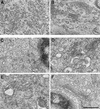
Cell-free assays that mimic the disassembly and reassembly cycle of the Golgi apparatus during mitosis implicated GRASP65 as a mitotically regulated stacking factor. We now present evidence that GRASP65 is directly involved in stacking Golgi cisternae. GRASP65 is the major phosphorylation target in rat liver Golgi membranes of two mitotic kinases, cdc2-cyclin B and polo-like kinases, which alone will unstack Golgi membranes, generating single cisternae. Mitotic cells microinjected with antibodies to GRASP65 fail to form proper Golgi stacks after cell division. Beads coated with GRASP65 homodimers form extensive aggregates consistent with the formation of trans oligomers. These can be disaggregated using purified cdc2-cyclin B1 and polo-like kinases, and re-aggregated after dephosphorylation of GRASP65. Together, these data demonstrate that GRASP65 has the properties required to bind surfaces together in a mitotically regulated manner.
Figures


References
-
- Barr F.A., Puype,M., Vandekerckhove,J. and Warren,G. (1997) GRASP65, a protein involved in the stacking of Golgi cisternae. Cell, 91, 253–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

