A role for maternal beta-catenin in early mesoderm induction in Xenopus
- PMID: 12839992
- PMCID: PMC165652
- DOI: 10.1093/emboj/cdg328
A role for maternal beta-catenin in early mesoderm induction in Xenopus
Abstract
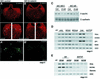
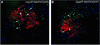
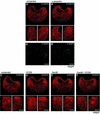
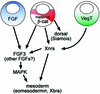
Mesoderm formation results from an inducing process that requires maternal and zygotic FGF/MAPK and TGFbeta activities, while maternal activation of the Wnt/beta-catenin pathway determines the anterior-dorsal axis. Here, we show a new role of Wnt/beta-catenin signaling in mesoderm induction. We find that maternal beta-catenin signaling is not only active dorsally but also all around the equatorial region, coinciding with the prospective mesoderm. Maternal beta-catenin function is required both for expression of dorsal genes and for activation of MAPK and the mesodermal markers Xbra and eomesodermin. beta-catenin acts in a non- cell-autonomous manner upstream of zygotic FGF and nodal signals. The Wnt/beta-catenin activity in the equatorial region of the early embryo is the first example of a maternally provided mesoderm inducer restricted to the prospective mesoderm.
Figures



References
-
- Amaya E., Musci,T.J. and Kirschner,M.W. (1991) Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell, 66, 257–270. - PubMed
-
- Angerer L.M. and Angerer,R.C. (2000) Animal–vegetal axis patterning mechanisms in the early sea urchin embryo. Dev. Biol., 218, 1–12. - PubMed
-
- Cadigan K. and Nusse,R. (1997) Wnt signaling: a common theme in animal development. Genes Dev., 11, 3286–3305. - PubMed
-
- Campione M. et al. (1999) The homeobox gene Pitx2: mediator of asymmetric left–right signaling in vertebrate heart and gut looping. Development, 126, 1225–1234. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

