The oncogenic RAS2(val19) mutation locks respiration, independently of PKA, in a mode prone to generate ROS
- PMID: 12839995
- PMCID: PMC165639
- DOI: 10.1093/emboj/cdg314
The oncogenic RAS2(val19) mutation locks respiration, independently of PKA, in a mode prone to generate ROS
Erratum in
- EMBO J. 2003 Sep 1;22(17):4577
Abstract
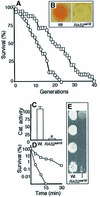
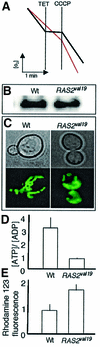
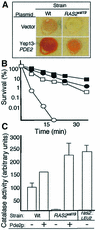
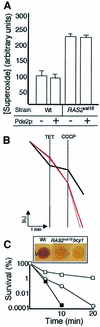
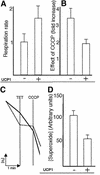
The RAS2(val19) allele, which renders the cAMP-PKA pathway constitutively active and decreases the replicative life-span of yeast cells, is demonstrated to increase production of reactive oxygen species (ROS) and to elevate oxidative protein damage. Mitochondrial respiration in the mutant is locked in a non-phosphorylating mode prone to generate ROS but this phenotype is not linked to a constitutively active PKA pathway. In contrast, providing RAS2(val19) cells with the mammalian uncoupling protein UCP1 restores phosphorylating respiration and reduces ROS levels, but does not correct for PKA-dependent defects. Thus, the RAS2(val19) allele acts like a double-edged sword with respect to oxidation management: (i). it diminishes expression of STRE element genes required for oxidative stress defenses in a PKA-dependent fashion, and (ii). it affects endogenous ROS production and the respiratory state in a PKA-independent way. The effect of the oncogenic RAS allele on the replicative life-span is primarily asserted via the PKA-dependent pathway since Pde2p, but not UCP1, overproduction suppressed premature aging of the RAS2(val19) mutant.
Figures

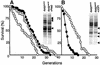
References
-
- Aguilaniu H., Gustafsson,L., Rigoulet,M. and Nystrom,T. (2003) Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science, 299, 1751–1753. - PubMed
-
- Aguilaniu H., Gustafsson,L., Rigoulet,M. and Nystrom,T. (2001) Protein oxidation in G0 cells of Saccharomyces cerevisiae depends on the state rather than rate of respiration and is enhanced in pos9 but not yap1 mutants. J. Biol. Chem., 28, 28. - PubMed
-
- Bergmeyer H. (1974) Methods in Enzymatic Analysis. Academic Press, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

