An ADAR that edits transcripts encoding ion channel subunits functions as a dimer
- PMID: 12840004
- PMCID: PMC165651
- DOI: 10.1093/emboj/cdg327
An ADAR that edits transcripts encoding ion channel subunits functions as a dimer
Abstract
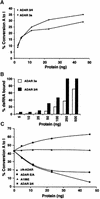
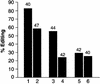
In this report, we establish that Drosophila ADAR (adenosine deaminase acting on RNA) forms a dimer on double-stranded (ds) RNA, a process essential for editing activity. The minimum region required for dimerization is the N-terminus and dsRNA-binding domain 1 (dsRBD1). Single point mutations within dsRBD1 abolish RNA-binding activity and dimer formation. These mutations and glycerol gradient analysis indicate that binding to dsRNA is important for dimerization. However, dimerization can be uncoupled from dsRNA-binding activity, as a deletion of the N-terminus (amino acids 1-46) yields a monomeric ADAR that retains the ability to bind dsRNA but is inactive in an editing assay, demonstrating that ADAR is only active as a dimer. Different isoforms of ADAR with different editing activities can form heterodimers and this can have a significant effect on editing in vitro as well as in vivo. We propose a model for ADAR dimerization whereby ADAR monomers first contact dsRNA; however, it is only when the second monomer binds and a dimer is formed that deamination occurs.
Figures





Similar articles
-
dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing.RNA. 2000 Jul;6(7):1004-18. doi: 10.1017/s1355838200000248. RNA. 2000. PMID: 10917596 Free PMC article.
-
ADAR RNA editing below the backbone.RNA. 2017 Sep;23(9):1317-1328. doi: 10.1261/rna.060921.117. Epub 2017 May 30. RNA. 2017. PMID: 28559490 Free PMC article. Review.
-
A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains.RNA. 2000 May;6(5):755-67. doi: 10.1017/s1355838200000170. RNA. 2000. PMID: 10836796 Free PMC article.
-
Identification and expression profiles of ADAR1 gene, responsible for RNA editing, in responses to dsRNA and GCRV challenge in grass carp (Ctenopharyngodon idella).Fish Shellfish Immunol. 2012 Oct;33(4):1042-9. doi: 10.1016/j.fsi.2012.07.002. Epub 2012 Jul 14. Fish Shellfish Immunol. 2012. PMID: 22796906
-
Regulation and functions of ADAR in drosophila.Curr Top Microbiol Immunol. 2012;353:221-36. doi: 10.1007/82_2011_152. Curr Top Microbiol Immunol. 2012. PMID: 21761288 Review.
Cited by
-
Genomic analysis of ADAR1 binding and its involvement in multiple RNA processing pathways.Nat Commun. 2015 Mar 9;6:6355. doi: 10.1038/ncomms7355. Nat Commun. 2015. PMID: 25751603 Free PMC article.
-
Tuning of RNA editing by ADAR is required in Drosophila.EMBO J. 2005 Jun 15;24(12):2183-93. doi: 10.1038/sj.emboj.7600691. Epub 2005 May 26. EMBO J. 2005. PMID: 15920480 Free PMC article.
-
Dissecting the splicing mechanism of the Drosophila editing enzyme; dADAR.Nucleic Acids Res. 2009 Apr;37(5):1663-71. doi: 10.1093/nar/gkn1080. Epub 2009 Jan 19. Nucleic Acids Res. 2009. Retraction in: Nucleic Acids Res. 2024 May 22;52(9):5421. doi: 10.1093/nar/gkae221. PMID: 19153139 Free PMC article. Retracted.
-
Solution structure of the N-terminal dsRBD of Drosophila ADAR and interaction studies with RNA.Biochimie. 2012 Jul;94(7):1499-509. doi: 10.1016/j.biochi.2011.12.017. Epub 2011 Dec 23. Biochimie. 2012. PMID: 22210494 Free PMC article.
-
Activity-regulated RNA editing in select neuronal subfields in hippocampus.Nucleic Acids Res. 2013 Jan;41(2):1124-34. doi: 10.1093/nar/gks1045. Epub 2012 Nov 20. Nucleic Acids Res. 2013. PMID: 23172290 Free PMC article.
References
-
- Betts L., Xiang,S., Short,S.A., Wolfenden,R. and Carter,C.W.Jr (1994) Cytidine deaminase. The 2.3 A crystal structure of an enzyme: transition-state analog complex. J. Mol. Biol., 235, 635–656. - PubMed
-
- Burns C.M., Chu,H., Rueter,S.M., Hutchinson,L.K., Canton,H., Sanders-Bush,E. and Emeson,R.B. (1997) Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature, 387, 303–308. - PubMed
-
- Caceres J.F. and Kornblihtt,A.R. (2002) Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet., 18, 186–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

