Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth
- PMID: 12840060
- PMCID: PMC162282
- DOI: 10.1172/JCI16645
Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth
Abstract
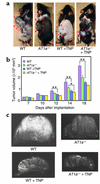
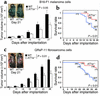
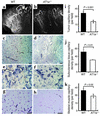
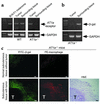
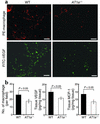
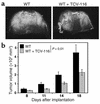
Although the renin angiotensin system (RAS) is a major regulator of vascular homeostasis, the role of the RAS in tumor angiogenesis is little understood. Here we show that host angiotensin II (ATII) type 1 (AT1) receptor plays an important role in angiogenesis and growth of tumor cells engrafted in mice. Subcutaneous B16-F1 melanoma-induced angiogenesis as assessed by tissue capillary density and microangiography was prominent in WT mice but was reduced in AT1a receptor-deficient (AT1a-/-) mice. Consequently, tumor growth rate was significantly slower, and the mouse survival rate was greater, in AT1a-/- mice than in WT mice. Tumor growth was also reduced in WT mice treated with TCV-116, a selective blocker of AT1 receptor. Because the beta-galactosidase gene was inserted into the AT1a gene locus in AT1a-/- mice, the site of beta-galactosidase expression represents the AT1a receptor expression in these mutant mice. In tumor-implanted AT1a-/- mice, the major site of the beta-galactosidase expression was macrophages in tissues surrounding tumors. Moreover, the number of infiltrated macrophages was significantly lower in AT1a-/- mice than in WT mice, and double-immunofluorescence staining revealed that these macrophages expressed VEGF protein intensively. Therefore, the host ATII-AT1 receptor pathway supports tumor-associated macrophage infiltration, which results in enhanced tissue VEGF protein levels. The host ATII-AT1 receptor pathway thereby plays important roles in tumor-related angiogenesis and growth in vivo.
Figures
References
-
- Brunner HR, et al. Essential hypertension: rennin and aldosterone, heart attack and stroke. N. Engl. J. Med. 1972;286:441–449. - PubMed
-
- Lever AF, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

