Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice
- PMID: 12840062
- PMCID: PMC162290
- DOI: 10.1172/JCI17845
Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice
Abstract
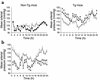
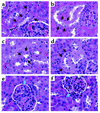
Obesity is closely associated with the metabolic syndrome, a combination of disorders including insulin resistance, diabetes, dyslipidemia, and hypertension. A role for local glucocorticoid reamplification in obesity and the metabolic syndrome has been suggested. The enzyme 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) regenerates active cortisol from inactive 11-keto forms, and aP2-HSD1 mice with relative transgenic overexpression of this enzyme in fat cells develop visceral obesity with insulin resistance and dyslipidemia. Here we report that aP2-HSD1 mice also have high arterial blood pressure (BP). The mice have increased sensitivity to dietary salt and increased plasma levels of angiotensinogen, angiotensin II, and aldosterone. This hypertension is abolished by selective angiotensin II receptor AT-1 antagonist at a low dose that does not affect BP in non-Tg littermates. These findings suggest that activation of the circulating renin-angiotensin system (RAS) develops in aP2-HSD1 mice. The long-term hypertension is further reflected by an appreciable hypertrophy and hyperplasia of the distal tubule epithelium of the nephron, resembling salt-sensitive or angiotensin II-mediated hypertension. Taken together, our findings suggest that overexpression of 11beta-HSD1 in fat is sufficient to cause salt-sensitive hypertension mediated by an activated RAS. The potential role of adipose 11beta-HSD1 in mediating critical features of the metabolic syndrome extends beyond obesity and metabolic complications to include the most central cardiovascular feature of this disorder.
Figures
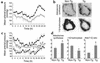

References
-
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. - PubMed
-
- Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contributions of adipocytokines, adipocyte-derived bioactive substances. Ann. N. Y. Acad. Sci. 1999;892:146–154. - PubMed
-
- Busetto L. Visceral obesity and the metabolic syndrome. Effect of weight loss. Nutr. Metab. Cardiovasc. Dis. 2001;11:195–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

