Expansion of human SCID-repopulating cells under hypoxic conditions
- PMID: 12840067
- PMCID: PMC162287
- DOI: 10.1172/JCI17669
Expansion of human SCID-repopulating cells under hypoxic conditions
Abstract
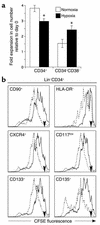
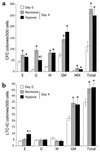
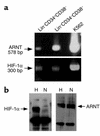
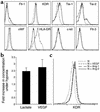
It has been proposed that bone marrow (BM) hematopoietic stem and progenitor cells are distributed along an oxygen (O2) gradient, where stem cells reside in the most hypoxic areas and proliferating progenitors are found in O2-rich areas. However, the effects of hypoxia on human hematopoietic stem cells (HSCs) have not been characterized. Our objective was to evaluate the functional and molecular responses of human BM progenitors and stem cells to hypoxic conditions. BM lineage-negative (Lin-) CD34+CD38- cells were cultured in serum-free medium under 1.5% O2 (hypoxia) or 20% O2 (normoxia) for 4 days. Using limiting dilution analysis, we demonstrate that the absolute number of SCID-repopulating cells (SRCs) increased by 5.8-fold in hypoxic cultures compared with normoxia, and by 4.2-fold compared with freshly isolated Lin-CD34+CD38- cells. The observed increase in BM-repopulating activity was associated with a preferential expansion of Lin-CD34+CD38- cells. We also demonstrate that, in response to hypoxia, hypoxia-inducible factor-1alpha protein was stabilized, surface expression of angiogenic receptors was upregulated, and VEGF secretion increased in BM Lin-CD34+ cultures. The use of low O2 levels to enhance the survival and/or self-renewal of human BM HSCs in vitro represents an important advance and could have valuable clinical implications.
Figures

References
-
- Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. - PubMed
-
- Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp. Hematol. 1981;9:391–410. - PubMed
-
- Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. - PubMed
-
- Ivanovic Z, et al. Incubation of murine bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br. J. Haematol. 2000;108:424–429. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

