The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis
- PMID: 12840145
- PMCID: PMC166386
- DOI: 10.1073/pnas.1433065100
The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis
Abstract
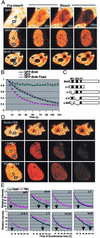
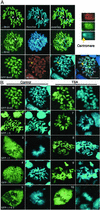
Previous in vitro studies showed that the bromodomain binds to acetyllysines on histone tails, leading to the proposal that the domain is involved in deciphering the histone code. However, there is little in vivo evidence supporting the binding of bromodomains to acetylated chromatin in the native environment. Brd4 is a member of the BET family that carries two bromodomains. It associates with mitotic chromosomes, a feature characteristic of the family. Here, we studied the interaction of Brd4 with chromatin in living cells by photobleaching. Brd4 was mobile and interacted with chromatin with a rapid "on and off" mode of binding. This interaction required both bromodomains. Indicating a preferential interaction with acetylated chromatin, Brd4 became less mobile upon increased chromatin acetylation caused by a histone deacetylase inhibitor. Providing biochemical support, salt solubility of Brd4 was markedly reduced upon increased histone acetylation. This change also required both bromodomains. In peptide binding assays, Brd4 avidly bound to di- and tetraacetylated histone H4 and diacetylated H3, but weakly or not at all to mono- and unacetylated H3 and H4. By contrast, it did not bind to unacetylated H4 or H3. Further, Brd4 colocalized with acetylated H4 and H3 in noncentromeric regions of mitotic chromosomes. This colocalization also required both bromodomains. These observations indicate that Brd4 specifically recognizes acetylated histone codes, and this recognition is passed onto the chromatin of newly divided cells.
Figures


References
-
- Strahl, B. D. & Allis, C. D. (2000) Nature 403, 41–45. - PubMed
-
- Jenuwein, T. & Allis, C. D. (2001) Science 293, 1074–1080. - PubMed
-
- Jeanmougin, F., Wurtz, J. M., Le Douarin, B., Chambon, P. & Losson, R. (1997) Trends Biochem. Sci. 22, 151–153. - PubMed
-
- Dhalluin, C., Carlson, J. E., Zeng, L., He, C., Aggarwal, A. K. & Zhou, M. M. (1999) Nature 399, 491–496. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

