Werner syndrome protein limits MYC-induced cellular senescence
- PMID: 12842909
- PMCID: PMC196129
- DOI: 10.1101/gad.1100303
Werner syndrome protein limits MYC-induced cellular senescence
Abstract
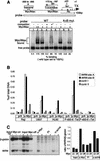
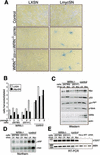
The MYC oncoprotein is a transcription factor that coordinates cell growth and division. MYC overexpression exacerbates genomic instability and sensitizes cells to apoptotic stimuli. Here we demonstrate that MYC directly stimulates transcription of the human Werner syndrome gene, WRN, which encodes a conserved RecQ helicase. Loss-of-function mutations in WRN lead to genomic instability, an elevated cancer risk, and premature cellular senescence. The overexpression of MYC in WRN syndrome fibroblasts or after WRN depletion from control fibroblasts led to rapid cellular senescence that could not be suppressed by hTERT expression. We propose that WRN up-regulation by MYC may promote MYC-driven tumorigenesis by preventing cellular senescence.
Figures


References
-
- Adams J.M., Harris, A.W., Pinkert, C.A., Corcoran, L.M., Alexander, W.S., Cory, S., Palmiter, R.D., and Brinster, R.L. 1985. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318: 533–538. - PubMed
-
- Brosh R.M. and Bohr, V.A. 2002. Roles of the Werner syndrome protein in pathways required for maintenance of genome stability. Exp. Gerontol. 37: 491–506. - PubMed
-
- Brown J.P., Wei, W., and Sedivy, J.M. 1997. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277: 831–834. - PubMed
-
- Brummelkamp T.R., Bernards, R., and Agami, R. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
