cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2
- PMID: 12842910
- PMCID: PMC196130
- DOI: 10.1101/gad.1097103
cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2
Abstract
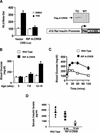
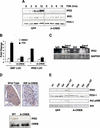
The incretin hormone GLP1 promotes islet-cell survival via the second messenger cAMP. Here we show that mice deficient in the activity of CREB, caused by expression of a dominant-negative A-CREB transgene in pancreatic beta-cells, develop diabetes secondary to beta-cell apoptosis. Remarkably, A-CREB severely disrupted expression of IRS2, an insulin signaling pathway component that is shown here to be a direct target for CREB action in vivo. As induction of IRS2by cAMP enhanced activation of the survival kinase Akt in response to insulin and IGF-1, our results demonstrate a novel mechanism by which opposing pathways cooperate in promoting cell survival.
Figures


References
-
- Bonner-Weir S., Deery, D., Leahy, J.L., and Weir, G.C. 1989. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes 38: 49–53. - PubMed
-
- Bruning J.C., Winnay, J., Bonner-Weir, S., Taylor, S.I., Accili, D., and Kahn, C.R. 1997. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88: 561–572. - PubMed
-
- Canettieri G., Morantte, I., Guzman, E., Asahara, H., Herzig, S., Anderson, S.D., Yates, J.R., and Montminy, M. 2003. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat. Struct. Biol. 10: 175–181. - PubMed
-
- Conkright M.D., Guzmán, E., Flechner, L., Su, A.I., Hogenesch, J., and Montminy, M. 2003. Genome wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol. Cell 11: 1101–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
