Cell fate decisions within the mouse organizer are governed by graded Nodal signals
- PMID: 12842913
- PMCID: PMC196136
- DOI: 10.1101/gad.1100503
Cell fate decisions within the mouse organizer are governed by graded Nodal signals
Abstract
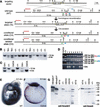
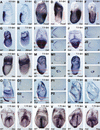
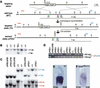
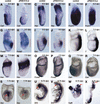
It is well known that cell fate decisions in the mouse organizer region during gastrulation ultimately govern gut formation and patterning, left-right axis determination, and development of the central nervous system. Previous studies suggest that signaling pathways activated by Nodal, bone morphogenetic protein (BMP), and Wnt ligands coordinately regulate patterning of the streak and the formation of midline organizing tissues, but the specific contributions of these molecules within discrete cell lineages are poorly defined. Here we removed Smad2 activity in the epiblast, using a conditional inactivation strategy. Abrogation of Smad2 does not compromise primitive streak (PS) formation or gastrulation movements, but rather results in a failure to correctly specify the anterior definitive endoderm (ADE) and prechordal plate (PCP) progenitors. To selectively lower Nodal activity in the posterior epiblast, we generated a novel allele lacking the proximal epiblast enhancer (PEE) governing Nodal expression in the PS. As for conditional inactivation of Smad2, germ-line deletion of the PEE selectively disrupts development of the anterior streak. In striking contrast, the node and its midline derivatives, the notochord and floor plate, develop normally in both categories of mutant embryos. Finally, we show that removal of one copy of Smad3 in the context of a Smad2-deficient epiblast results in a failure to specify all axial midline tissues. These findings conclusively demonstrate that graded Nodal/Smad2 signals govern allocation of the axial mesendoderm precursors that selectively give rise to the ADE and PCP mesoderm.
Figures



References
-
- Ang S.L. and Rossant, J. 1994. HNF-3 β is essential for node and notochord formation in mouse development. Cell 78: 561–574. - PubMed
-
- Ang S.L., Conlon, R.A., Jin, O., and Rossant, J. 1994. Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development 120: 2979–2989. - PubMed
-
- Bachiller D., Klingensmith, J., Kemp, C., Belo, J.A., Anderson, R.M., May, S.R., McMahon, J.A., McMahon, A.P., Harland, R.M., Rossant, J., et al. 2000. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403: 658–661. - PubMed
-
- Beddington R.S. and Robertson, E.J. 1999. Axis development and early asymmetry in mammals. Cell 96: 195–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
