Real-time PCR-based system for simultaneous quantification of human papillomavirus types associated with high risk of cervical cancer
- PMID: 12843067
- PMCID: PMC165384
- DOI: 10.1128/JCM.41.7.3221-3228.2003
Real-time PCR-based system for simultaneous quantification of human papillomavirus types associated with high risk of cervical cancer
Abstract
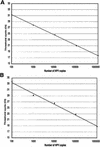
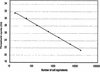
We have previously shown that women with a high titer of human papillomavirus type 16 (HPV16) in cervical epithelial cells have an increased risk of developing cervical carcinoma in situ. In order to study the relationship between viral DNA amount and risk of cervical carcinoma for the HPV types most commonly found in cervical tumors, we developed a real-time PCR assay for the detection and quantification of HPV16, -18, -31, -33, -35, -39, -45, -52, -58, and -67. These HPV types are analyzed in two reaction tubes, allowing for independent quantification of three viral types, or groups of viral types, in each reaction. A separate reaction is used for estimating the number of a nuclear single-copy gene and is used to calculate the HPV copy number per genomic DNA equivalent in the sample. The system has a dynamic range from 10(2) to 10(7) HPV copies per assay and is applicable to both fresh clinical samples and DNA extracted from archival samples. Reconstitution experiments, made to mimic infections with several HPV types, shows that individual HPV types can be detected in a mixture as long as they represent 1 to 10% of the main type. The system was evaluated with respect to technical specificity and sensitivity, reproducibility, reagent stability, and sample preparation protocol and then used to analyze clinical samples. This homogeneous assay provides a fast and sensitive way for estimating the viral load of a series of the most frequent oncogenic HPV types in biopsies, as well as cervical smear samples.
Figures




References
-
- Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, et al. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87:796-802. - PubMed
-
- Chua, K. L., and A. Hjerpe. 1995. Polymerase chain reaction analysis of human papillomavirus in archival cervical cytologic smears. Anal. Quant. Cytol. Histol. 17:221-229. - PubMed
-
- Higuchi, R., C. Fockler, G. Dollinger, and R. Watson. 1993. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Bio/Technology 11:1026-1030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

