Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons
- PMID: 12843211
- PMCID: PMC2343279
- DOI: 10.1113/jphysiol.2003.047357
Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons
Abstract
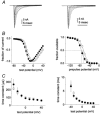
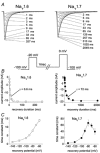
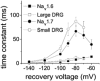
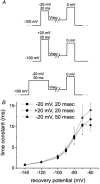
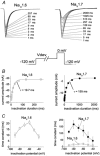
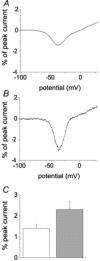
While large, myelinated dorsal root ganglion (DRG) neurons are capable of firing at high frequencies, small unmyelinated DRG neurons typically display much lower maximum firing frequencies. However, the molecular basis for this difference has not been delineated. Because the sodium currents in large DRG neurons exhibit rapid repriming (recovery from inactivation) kinetics and the sodium currents in small DRG neurons exhibit predominantly slow repriming kinetics, it has been proposed that differences in sodium channels might contribute to the determination of repetitive firing properties in DRG neurons. A recent study demonstrated that Nav1.7 expression is negatively correlated with conduction velocity and DRG cell size, while the Nav1.6 voltage-gated sodium channel has been implicated as the predominant isoform present at nodes of Ranvier of myelinated fibres. Therefore we characterized and compared the functional properties, including repriming, of recombinant Nav1.6 and Nav1.7 channels expressed in mouse DRG neurons. Both Nav1.6 and Nav1.7 channels generated fast-activating and fast-inactivating currents. However recovery from inactivation was significantly faster (approximately 5-fold at -70 mV) for Nav1.6 currents than for Nav1.7 currents. The recovery from inactivation of Nav1.6 channels was also much faster than that of native tetrodotoxin-sensitive sodium currents recorded from small spinal sensory neurons, but similar to that of tetrodotoxin-sensitive sodium currents recorded from large spinal sensory neurons. Development of closed-state inactivation was also much faster for Nav1.6 currents than for Nav1.7 currents. Our results indicate that the firing properties of DRG neurons can be tuned by regulating expression of different sodium channel isoforms that have distinct repriming and closed-state inactivation kinetics.
Figures
References
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel Nav1. 8 has a specialized function in pain pathways. Nature Neurosci. 1999;2:541–548. - PubMed
-
- Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999;82:2776–2785. - PubMed
-
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Brain Res Mol Brain Res. 1996;43:117–132. - PubMed
-
- Black JA, Waxman SG. Sodium channel expression: a dynamic process in neurons and non-neuronal cells. Dev Neurosci. 1996;18:139–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

