Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions
- PMID: 12843238
- PMCID: PMC6741224
- DOI: 10.1523/JNEUROSCI.23-13-05393.2003
Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions
Abstract
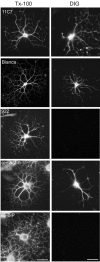
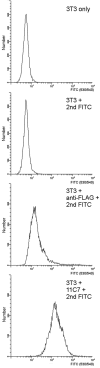
Nogo-A is a potent neurite growth inhibitor in vitro and plays a role both in the restriction of axonal regeneration after injury and in structural plasticity in the CNS of higher vertebrates. The regions that mediate inhibition and the topology of the molecule in the plasma membrane have to be defined. Here we demonstrate the presence of three different active sites: (1) an N-terminal region involved in the inhibition of fibroblast spreading, (2) a stretch encoded by the Nogo-A-specific exon that restricts neurite outgrowth and cell spreading and induces growth cone collapse, and (3) a C-terminal region (Nogo-66) with growth cone collapsing function. We show that Nogo-A-specific active fragments bind to the cell surface of responsive cells and to rat brain cortical membranes, suggesting the existence of specific binding partners or receptors. Several antibodies against different epitopes on the Nogo-A-specific part of the protein as well as antisera against the 66 aa loop in the C-terminus stain the cell surface of living cultured oligodendrocytes. Nogo-A is also labeled by nonmembrane-permeable biotin derivatives applied to living oligodendrocyte cultures. Immunofluorescent staining of intracellular, endoplasmic reticulum-associated Nogo-A in cells after selective permeabilization of the plasma membrane reveals that the epitopes of Nogo-A, shown to be accessible at the cell surface, are exposed to the cytoplasm. This suggests that Nogo-A could have a second membrane topology. The two proposed topological variants may have different intracellular as well as extracellular functions.
Figures
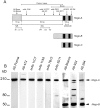

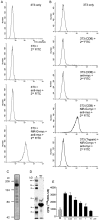
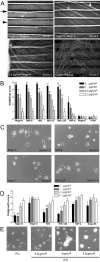
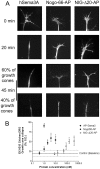

Similar articles
-
Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1.J Neurosci. 2002 Dec 1;22(23):10368-76. doi: 10.1523/JNEUROSCI.22-23-10368.2002. J Neurosci. 2002. PMID: 12451136 Free PMC article.
-
Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1.Nature. 2000 Jan 27;403(6768):434-9. doi: 10.1038/35000219. Nature. 2000. PMID: 10667796
-
Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin.J Neurosci. 2002 Oct 15;22(20):8876-83. doi: 10.1523/JNEUROSCI.22-20-08876.2002. J Neurosci. 2002. PMID: 12388594 Free PMC article.
-
[Inhibitory proteins against axon regeneration in the central nervous system].Sheng Li Ke Xue Jin Zhan. 2004 Oct;35(4):311-4. Sheng Li Ke Xue Jin Zhan. 2004. PMID: 15727207 Review. Chinese.
-
The Nogo-66 receptor: focusing myelin inhibition of axon regeneration.Trends Neurosci. 2003 Apr;26(4):193-8. doi: 10.1016/S0166-2236(03)00062-6. Trends Neurosci. 2003. PMID: 12689770 Review.
Cited by
-
No go for brain tumors?J Mol Neurosci. 2005;25(1):1-6. doi: 10.1385/jmn:25:1:001. J Mol Neurosci. 2005. PMID: 15781961 Review.
-
Recapitulate development to promote axonal regeneration: good or bad approach?Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1565-74. doi: 10.1098/rstb.2006.1885. Philos Trans R Soc Lond B Biol Sci. 2006. PMID: 16939975 Free PMC article. Review.
-
T cells can mediate viral clearance from ependyma but not from brain parenchyma in a major histocompatibility class I- and perforin-independent manner.Brain. 2010 Apr;133(Pt 4):1054-66. doi: 10.1093/brain/awq028. Epub 2010 Mar 30. Brain. 2010. PMID: 20354003 Free PMC article.
-
The reticulons: a family of proteins with diverse functions.Genome Biol. 2007;8(12):234. doi: 10.1186/gb-2007-8-12-234. Genome Biol. 2007. PMID: 18177508 Free PMC article. Review.
-
Myelin-associated proteins block the migration of olfactory ensheathing cells: an in vitro study using single-cell tracking and traction force microscopy.Cell Mol Life Sci. 2012 May;69(10):1689-703. doi: 10.1007/s00018-011-0893-1. Epub 2011 Dec 29. Cell Mol Life Sci. 2012. PMID: 22205212 Free PMC article.
References
-
- Amberger VR, Hensel T, Ogata N, Schwab ME ( 1998) Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res 58: 149–158. - PubMed
-
- Bandtlow CE, Löschinger J ( 1997) Developmental changes in neuronal responsiveness to the CNS myelin-associated neurite growth inhibitor NI-35/250. Eur J Neurosci 9: 2743–2752. - PubMed
-
- Bandtlow CE, Schmidt MF, Hassinger TD, Schwab ME, Kater SB ( 1993) Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science 259: 80–83. - PubMed
-
- Behar O, Mizuno K, Neumann S, Woolf CJ ( 2000) Putting the spinal cord together again. Neuron 26: 291–293. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
