Genetic analysis of the roles of Hh, FGF8, and nodal signaling during catecholaminergic system development in the zebrafish brain
- PMID: 12843251
- PMCID: PMC6741235
- DOI: 10.1523/JNEUROSCI.23-13-05507.2003
Genetic analysis of the roles of Hh, FGF8, and nodal signaling during catecholaminergic system development in the zebrafish brain
Abstract
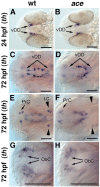
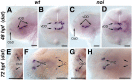
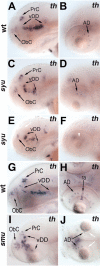
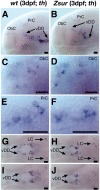
CNS catecholaminergic neurons can be distinguished by their neurotransmitters as dopaminergic or noradrenergic and form in distinct regions at characteristic embryonic stages. This raises the question of whether all catecholaminergic neurons of one transmitter type are specified by the same set of factors. Therefore, we performed genetic analyses to define signaling requirements for the specification of distinct clusters of catecholaminergic neurons in zebrafish. In mutants affecting midbrain- hindbrain boundary (MHB) organizer formation, the earliest ventral diencephalic dopaminergic neurons appear normal. However, after 2 d of development, we observed fewer cells than in wild types, which suggests that the MHB provides proliferation or survival factors rather than specifying ventral diencephalic dopaminergic clusters. In hedgehog (Hh) pathway mutants, the formation of catecholaminergic neurons is affected only in the pretectal cluster. Surprisingly, neither fibroblast growth factor 8 (FGF8) alone nor in combination with Hh signaling is required for specification of early developing dopaminergic neurons. We analyzed the formation of prosomeric territories in the forebrain of Hh and Nodal pathway mutants to determine whether the absence of specific dopaminergic clusters may be caused by early patterning defects ablating corresponding parts of the CNS. In Nodal pathway mutants, ventral diencephalic and pretectal catecholaminergic neurons fail to develop, whereas both anatomical structures form at least in part. This suggests that Nodal signaling is required for catecholaminergic neuron specification. In summary, our results do not support the previously suggested dominant roles for sonic hedgehog and Fgf8 in specification of the first catecholaminergic neurons, but instead indicate a novel role for Nodal signaling in this process.
Figures





Similar articles
-
Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis.Development. 2004 Aug;131(15):3681-92. doi: 10.1242/dev.01235. Epub 2004 Jun 30. Development. 2004. PMID: 15229178
-
Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish.J Comp Neurol. 2010 Feb 15;518(4):439-58. doi: 10.1002/cne.22214. J Comp Neurol. 2010. PMID: 20017210 Free PMC article.
-
Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon.Development. 2006 Mar;133(5):855-64. doi: 10.1242/dev.02248. Epub 2006 Feb 1. Development. 2006. PMID: 16452095
-
Induction and specification of midbrain dopaminergic cells: focus on SHH, FGF8, and TGF-beta.Cell Tissue Res. 2004 Oct;318(1):23-33. doi: 10.1007/s00441-004-0916-4. Epub 2004 Aug 19. Cell Tissue Res. 2004. PMID: 15322912 Review.
-
Morphogens as growth cone signalling molecules.Brain Res Brain Res Rev. 2005 Sep;49(2):242-52. doi: 10.1016/j.brainresrev.2004.10.004. Epub 2004 Dec 24. Brain Res Brain Res Rev. 2005. PMID: 16111553 Review.
Cited by
-
Kapd Is Essential for Specification of the Dopaminergic Neurogenesis in Zebrafish Embryos.Mol Cells. 2021 Apr 30;44(4):233-244. doi: 10.14348/molcells.2021.0005. Mol Cells. 2021. PMID: 33820883 Free PMC article.
-
Wnt/β-catenin signaling promotes neurogenesis in the diencephalospinal dopaminergic system of embryonic zebrafish.Sci Rep. 2022 Jan 19;12(1):1030. doi: 10.1038/s41598-022-04833-8. Sci Rep. 2022. PMID: 35046434 Free PMC article.
-
Chemical and Genetic Zebrafish Models to Define Mechanisms of and Treatments for Dopaminergic Neurodegeneration.Int J Mol Sci. 2020 Aug 20;21(17):5981. doi: 10.3390/ijms21175981. Int J Mol Sci. 2020. PMID: 32825242 Free PMC article. Review.
-
Adult islet1 Expression Outlines Ventralized Derivatives Along Zebrafish Neuraxis.Front Neuroanat. 2019 Feb 26;13:19. doi: 10.3389/fnana.2019.00019. eCollection 2019. Front Neuroanat. 2019. PMID: 30863287 Free PMC article.
-
Zebrafish: a model system for the study of eye genetics.Prog Retin Eye Res. 2008 Jan;27(1):89-110. doi: 10.1016/j.preteyeres.2007.08.002. Epub 2007 Sep 7. Prog Retin Eye Res. 2008. PMID: 17962065 Free PMC article. Review.
References
-
- Belting HG, Hauptmann G, Meyer D, Abdelilah-Seyfried S, Chitnis A, Eschbach C, Soll I, Thisse C, Thisse B, Artinger KB, Lunde K, Driever W ( 2001) spiel ohne grenzen/pou2 is required during establishment of the zebrafish midbrain– hindbrain boundary organizer. Development 128: 4165–4176. - PMC - PubMed
-
- Brand M, Heisenberg CP, Jiang YJ, Beuchle D, Lun K, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C ( 1996) Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development 123: 179–190. - PubMed
-
- Briscoe J, Ericson J ( 1999) The specification of neuronal identity by graded Sonic Hedgehog signaling. Semin Cell Dev Biol 10: 353–362. - PubMed
-
- Briscoe J, Ericson J ( 2001) Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol 11: 43–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
