Action potential propagation in dendrites of rat mitral cells in vivo
- PMID: 12843256
- PMCID: PMC6741248
- DOI: 10.1523/JNEUROSCI.23-13-05553.2003
Action potential propagation in dendrites of rat mitral cells in vivo
Abstract
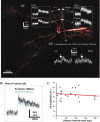
Odors evoke beta-gamma frequency field potential oscillations in the olfactory systems of awake and anesthetized vertebrates. In the rat olfactory bulb, these oscillations reflect the synchronous discharges of mitral cells that result from both their intrinsic membrane properties and their dendrodendritic interactions with local inhibitory interneurons. Activation of dendrodendritic synapses is purportedly involved in odor memory and odor contrast enhancement. Here we investigate in vivo to what extent action potentials propagate to remote dendrodendritic sites in the entire dendritic tree and if this propagation is changed during discharges at 40 Hz. By combining intracellular recording and two-photon microscopy imaging of intracellular calcium ([Ca2+]i), we show that in remote branches of the apical tuft and basal dendrites, transient Ca2+ changes are triggered by single sodium action potentials. Neither the amplitude of these Ca2+ transients nor that of action potentials obtained from intradendritic recordings showed a significant attenuation as a function of the distance from the soma. Calcium channel density seemed homogeneous; however, propagating action potentials occasionally failed to trigger a Ca2+ transient at a site closer to the soma whereas it did farther. This suggests that measurements of calcium transients underestimate the occurrence of sodium action potentials. During 40 Hz bursts of action potentials, [Ca2+]i increases with the number of action potentials in all dendritic compartments. These results suggest that the presence of release sites in dendrites is accompanied by an "axonal-like behavior" of the entire dendritic tree of mitral cells, including their most distal dendritic branches.
Figures





References
-
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT ( 2000) Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol 84: 1194–1203. - PubMed
-
- Chen WR, Shepherd GM ( 1997) Membrane and synaptic properties of mitral cells in slices of rat olfactory bulb. Brain Res 745: 189–196. - PubMed
-
- Chen WR, Midtgaard J, Shepherd GM ( 1997) Forward and backward propagation of dendritic impulses and their synaptic control in mitral cells. Science 278: 463–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
