Alteration of gene expression by chromosome loss in the postnatal mouse brain
- PMID: 12843262
- PMCID: PMC6741244
- DOI: 10.1523/JNEUROSCI.23-13-05599.2003
Alteration of gene expression by chromosome loss in the postnatal mouse brain
Abstract
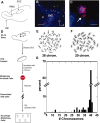
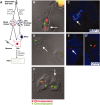
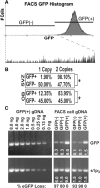
Frequent chromosomal aneuploidy has recently been discovered in normal neurons of the developing and mature murine CNS. Toward a more detailed understanding of aneuploidy and its effects on normal CNS cells, we examined the genomes of cells in the postnatal subventricular zone (SVZ), an area that harbors a large number of neural stem and progenitor cells (NPCs), which give rise to neurons and glia. Here we show that NPCs, neurons, and glia from the SVZ are frequently aneuploid. Karyotyping revealed that approximately 33% of mitotic SVZ cells lost or gained chromosomes in vivo, whereas interphase fluorescence in situ hybridization demonstrated aneuploidy in postnatal-born cells in the olfactory bulb (OB) in vivo, along with neurons, glia, and NPCs in vitro. One possible consequence of aneuploidy is altered gene expression through loss of heterozygosity (LOH). This was examined in a model of LOH: loss of transgene expression in mice hemizygous for a ubiquitously expressed enhanced green fluorescent protein (eGFP) transgene on chromosome 15. Concurrent examination of eGFP expression, transgene abundance, and chromosome 15 copy number demonstrated that a preponderance of living SVZ and OB cells not expressing eGFP lost one copy of chromosome 15; the eGFP transgene was lost in these cells as well. Although gene expression profiling revealed changes in expression levels of several genes relative to GFP-expressing controls, cells with LOH at chromosome 15 were morphologically normal and proliferated or underwent apoptosis at rates similar to those of euploid cells in vitro. These findings support the view that NPCs and postnatal-born neurons and glia can be aneuploid in vivo and functional gene expression can be permanently altered in living neural cells by chromosomal aneuploidy.
Figures



References
-
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY ( 2000) Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA 97: 12846–12851. - PMC - PubMed
-
- Barch MJ, Knudsen T, Spurbeck JL ( 1997) The AGT cytogenetics laboratory manual, Chap 3, Ed 3. Philadelphia: Lippincott–Raven.
-
- Chun J, Schatz DG ( 1999a) Developmental neurobiology: alternative ends for a familiar story? Curr Biol 9: R251–R253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
