Caspase-independent photoreceptor apoptosis in mouse models of retinal degeneration
- PMID: 12843276
- PMCID: PMC6741277
- DOI: 10.1523/JNEUROSCI.23-13-05723.2003
Caspase-independent photoreceptor apoptosis in mouse models of retinal degeneration
Abstract
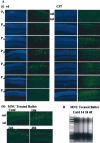
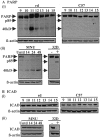
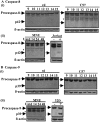
Apoptosis is the mode of cell death in retinitis pigmentosa, a group of retinal degenerative disorders primarily affecting rod photoreceptors. Although caspases have been demonstrated to play a central role in many incidences of apoptosis, accumulating evidence suggests that they may not be required for all forms of apoptotic cell death. The present study examined the mechanism of cell death in two in vivo models of photoreceptor apoptosis: the retinal degeneration (rd) mouse, a naturally occurring mutant model, and N-methyl-N-nitrosourea-induced retinal degeneration. Specifically, we examined the activation status of caspase-9, -8, -7, -3, and -2 and determined the caspase requirements for cytochrome c release, DNA fragmentation, and apoptosis-associated proteolysis of specific caspase substrates. We show that apoptosis in both in vivo models is independent of caspase-9, -8, -7, -3, and -2 activation. DNA fragmentation occurs in the absence of caspase-mediated ICAD (inhibitor of caspase-activated DNase) proteolysis, suggesting that an alternative endonuclease is responsible for DNA cleavage in these models. Importantly, we show that apoptosome activation is prevented because of an absence of mitochondrial cytochrome c release. Experiments performed using a cell-free system indicate that cytochrome c-dependent proteolysis and activation of caspase-9 can be restored in a neonatal cell-free system. However, we found that cytochrome c-dependent proteolysis and activation of caspase-9 could not be restored in an adult cell-free system because of an age-related decrease in the expression of Apaf-1 in the normal developing mouse retina. In the rd mouse, however, this age-related downregulation of apoptotic proteins was not observed, highlighting a critical feature of this model and the prevention of cytochrome c release as an apical event in caspase-independent apoptosis in this system.
Figures



Similar articles
-
Caspase-3 in postnatal retinal development and degeneration.Invest Ophthalmol Vis Sci. 2004 Mar;45(3):964-70. doi: 10.1167/iovs.03-0439. Invest Ophthalmol Vis Sci. 2004. PMID: 14985318
-
Lycium barbarum polysaccharides attenuates N-methy-N-nitrosourea-induced photoreceptor cell apoptosis in rats through regulation of poly (ADP-ribose) polymerase and caspase expression.J Ethnopharmacol. 2016 Sep 15;191:125-134. doi: 10.1016/j.jep.2016.05.037. Epub 2016 May 18. J Ethnopharmacol. 2016. PMID: 27208869
-
Activation of caspase-3 during degeneration of the outer nuclear layer in the rd mouse retina.Ophthalmic Res. 2002 May-Jun;34(3):150-7. doi: 10.1159/000063659. Ophthalmic Res. 2002. PMID: 12097798
-
[Characteristics of N-methyl-N-nitrosourea-induced retinal degeneration in animals and application for the therapy of human retinitis pigmentosa].Nippon Ganka Gakkai Zasshi. 2005 Jun;109(6):327-37. Nippon Ganka Gakkai Zasshi. 2005. PMID: 16047940 Review. Japanese.
-
Photoreceptor cell death mechanisms in inherited retinal degeneration.Mol Neurobiol. 2008 Dec;38(3):253-69. doi: 10.1007/s12035-008-8045-9. Epub 2008 Nov 4. Mol Neurobiol. 2008. PMID: 18982459 Review.
Cited by
-
Endoplasmic reticulum stress-associated cone photoreceptor degeneration in cyclic nucleotide-gated channel deficiency.J Biol Chem. 2012 May 25;287(22):18018-29. doi: 10.1074/jbc.M112.342220. Epub 2012 Apr 9. J Biol Chem. 2012. PMID: 22493484 Free PMC article.
-
A novel dual signaling axis for NSP 5a3a induced apoptosis in head and neck carcinoma.Oncotarget. 2011 Dec;2(12):1055-74. doi: 10.18632/oncotarget.306. Oncotarget. 2011. PMID: 22170762 Free PMC article.
-
Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse.J Neurosci. 2007 Sep 19;27(38):10311-9. doi: 10.1523/JNEUROSCI.1514-07.2007. J Neurosci. 2007. PMID: 17881537 Free PMC article.
-
Apoptosis and disease: a life or death decision.EMBO Rep. 2004 Jul;5(7):674-8. doi: 10.1038/sj.embor.7400191. Epub 2004 Jun 25. EMBO Rep. 2004. PMID: 15218528 Free PMC article.
-
RIP1 inhibition protects retinal ganglion cells in glaucoma models of ocular injury.Cell Death Differ. 2025 Feb;32(2):353-368. doi: 10.1038/s41418-024-01390-7. Cell Death Differ. 2025. PMID: 39448868 Free PMC article.
References
-
- Azarian SM, Williams DS ( 1995) Calpain activity in the retinas of normal and RCS rats. Curr Eye Res 14: 731–735. - PubMed
-
- Bascom RA, Manara S, Collins L, Molday RS, Kalnins VI, McInnes RR ( 1992) Cloning of the cDNA for a novel photoreceptor membrane protein (rom-1) identifies a disk rim protein family implicated in human retinopathies. Neuron 8: 1171–1184. - PubMed
-
- Borner C, Monney L ( 1999) Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ 6: 497–507. - PubMed
-
- Boulares AH, Zoltoski AJ, Contreras FJ, Yakovlev AG, Yoshihara K, Smulson ME ( 2002) Regulation of DNAS1L3 endonuclease activity by poly(ADP-ribosyl)ation during etoposide-induced apoptosis. Role of poly(ADP-ribose) polymerase-1 cleavage in endonuclease activation. J Biol Chem 277: 372–378. - PubMed
-
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB ( 1990) Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature 347: 677–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
