Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo
- PMID: 12843281
- PMCID: PMC6741236
- DOI: 10.1523/JNEUROSCI.23-13-05771.2003
Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo
Abstract
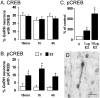
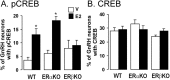
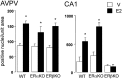
The gonadal steroid estrogen exerts an important modulatory influence on the activity of multiple neuronal networks. In addition to classical genomic mechanisms of action, estrogen also exerts poorly understood rapid, nongenomic effects on neurons. To examine whether estrogen may exert rapid actions on intracellular signaling within gonadotropin-releasing hormone (GnRH) neurons in vivo,we examined the phosphorylation status of cAMP response element-binding protein (CREB) in these cells after the administration of 17-beta-estradiol to ovariectomized (OVX) mice. The percentage of GnRH neurons expressing phosphorylated CREB was increased more than sixfold (p < 0.05) in a time- and dose-dependent manner by estrogen, with the increase first observed 15 min after estrogen administration. A series of in vitro studies demonstrated that estrogen acted directly on native GnRH neurons to phosphorylate CREB, but that estrogen conjugated to bovine serum albumin was without effect. The role of classical estrogen receptors (ERs) was evaluated using ER knock-out mice in vivo. The effect of estrogen on CREB phosphorylation in GnRH neurons was normal in ERalpha knock-out mice but completely absent in ERbeta knock-out mice. Finally, studies in intact female mice revealed levels of CREB phosphorylation within GnRH neurons that were equivalent to those of estrogen-treated OVX mice. These observations demonstrate that ERbeta mediates the rapid, direct effects of estrogen on the GnRH neuronal phenotype, and that these actions persist under physiological conditions. They also provide the first evidence for a role of ERbeta in nongenomic estrogen signaling within the brain in vivo.
Figures


References
-
- Benten WPM, Stephan C, Lieberherr M, Wunderlich F ( 2001) Estradiol signaling via sequestrable surface receptors. Endocrinology 142: 1669-1677. - PubMed
-
- Bronson FH ( 1981) The regulation of lutienizing hormone secretion by estrogen: relationship among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology 108: 506-516. - PubMed
-
- Cardona-Gomez G, Mendez P, Garcia-Segura LM ( 2002) Synergistic interaction of estradiol and insulin-like growth factor-1 in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Mol Brain Res 107: 80-88. - PubMed
-
- Carlstrom L, Ke ZJ, Unnerstall JR, Cohen RS, Pandey SC ( 2001) Estrogen modulation of the cyclic AMP response element-binding protein pathway. Effects of long-term and acute treatments. Neuroendocrinology 74: 227-243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
