Post-traumatic hyperexcitability is not caused by impaired buffering of extracellular potassium
- PMID: 12843291
- PMCID: PMC6741221
- DOI: 10.1523/JNEUROSCI.23-13-05865.2003
Post-traumatic hyperexcitability is not caused by impaired buffering of extracellular potassium
Abstract
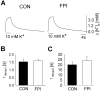
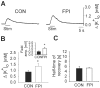
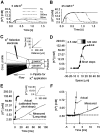
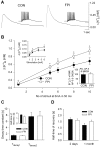
Impaired extracellular potassium buffering has been proposed as one of the major mechanisms underlying the increased risk for temporal lobe epilepsy after brain injury (D'Ambrosio et al., 1999). The present study systematically tested this hypothesis by measuring the resting [K+]o and recovery of the stimulation-evoked [K+]o increases in the dentate gyrus after experimental head trauma, using a combination of whole-cell recordings and ion-selective microelectrode recordings in rat hippocampal slices. Despite the presence of hyperexcitability, the resting [K+]o was not increased after injury. The faster rate of increase and larger amplitude of the orthodromically evoked [K+]o elevation after head trauma occurred in association with a greater population spike with shorter response latency. Contrary to the assumption in previous studies that the evoked activity in control and injured neuronal circuits is the same during antidromic activation, stimulation of granule cell axons in glutamate receptor antagonists evoked a greater [K+]o increase and a larger population spike. Although perforant path stimulation resulted in a larger [K+]o elevation after injury, the rate of clearance of the [K+]o transients evoked either by neuronal activity or by external application of potassium was not compromised. The [K+]o increase evoked by activation of the presynaptic afferents in isolation was not increased. In addition, the postsynaptic neuronal depolarization and firing evoked by exogenous potassium application was decreased after trauma. These results show that the regulation of [K+]o is not impaired after injury and indicate that the larger [K+]o increase evoked by neuronal activity is a consequence, rather than the primary mechanism underlying post-traumatic hyperexcitability.
Figures



Similar articles
-
Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the 'irritable mossy cell' hypothesis.J Physiol. 2000 Apr 1;524 Pt 1(Pt 1):117-34. doi: 10.1111/j.1469-7793.2000.00117.x. J Physiol. 2000. PMID: 10747187 Free PMC article.
-
Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus.J Neurophysiol. 2002 Jan;87(1):87-102. doi: 10.1152/jn.00240.2001. J Neurophysiol. 2002. PMID: 11784732
-
Cesium induces spontaneous epileptiform activity without changing extracellular potassium regulation in rat hippocampus.J Neurophysiol. 1999 Dec;82(6):3339-46. doi: 10.1152/jn.1999.82.6.3339. J Neurophysiol. 1999. PMID: 10601465
-
Neuronal interactions mediated by neurally evoked changes in the extracellular potassium concentration.J Exp Biol. 1984 Sep;112:179-97. doi: 10.1242/jeb.112.1.179. J Exp Biol. 1984. PMID: 6392467 Review.
-
Honey, I shrunk the extracellular space: Measurements and mechanisms of astrocyte swelling.Glia. 2022 Nov;70(11):2013-2031. doi: 10.1002/glia.24224. Epub 2022 May 30. Glia. 2022. PMID: 35635369 Free PMC article. Review.
Cited by
-
Distinct effect of impact rise times on immediate and early neuropathology after brain injury in juvenile rats.J Neurosci Res. 2014 Oct;92(10):1350-1361. doi: 10.1002/jnr.23401. Epub 2014 May 5. J Neurosci Res. 2014. PMID: 24799156 Free PMC article.
-
The impact of the glial spatial buffering on the K(+) Nernst potential.Cogn Neurodyn. 2011 Sep;5(3):285-91. doi: 10.1007/s11571-011-9165-x. Epub 2011 Jul 19. Cogn Neurodyn. 2011. PMID: 22942917 Free PMC article.
-
Monitoring hippocampus electrical activity in vitro on an elastically deformable microelectrode array.J Neurotrauma. 2009 Jul;26(7):1135-45. doi: 10.1089/neu.2008.0810. J Neurotrauma. 2009. PMID: 19594385 Free PMC article.
-
Single Neuron Modeling Identifies Potassium Channel Modulation as Potential Target for Repetitive Head Impacts.Neuroinformatics. 2023 Jul;21(3):501-516. doi: 10.1007/s12021-023-09633-7. Epub 2023 Jun 9. Neuroinformatics. 2023. PMID: 37294503 Free PMC article.
-
Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury.Nat Rev Neurol. 2010 Jul;6(7):393-403. doi: 10.1038/nrneurol.2010.74. Epub 2010 Jun 15. Nat Rev Neurol. 2010. PMID: 20551947 Free PMC article. Review.
References
-
- Aitken PG, Somjen GG ( 1986) The sources of extracellular potassium accumulation in the CA1 region of hippocampal slices. Brain Res 369: 163-167. - PubMed
-
- Annegers JF, Coan SP ( 2000) The risks of epilepsy after traumatic brain injury. Seizure 9: 453-457. - PubMed
-
- Ballanyi K, Grafe P, Reddy MM, ten Bruggencate G ( 1984) Different types of potassium transport linked to carbachol and γ-aminobutyric acid actions in rat sympathetic neurons. Neuroscience 12: 917-927. - PubMed
-
- Bordey A, Sontheimer H ( 1998) Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res 32: 286-303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
