Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy
- PMID: 12843297
- PMCID: PMC6741266
- DOI: 10.1523/JNEUROSCI.23-13-05928.2003
Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy
Abstract
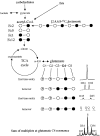
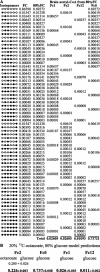
Glucose is the dominant oxidative fuel for brain, but studies have indicated that fatty acids are used by brain as well. We postulated that fatty acid oxidation in brain could contribute significantly to overall energy usage and account for non-glucose-derived energy production. [2,4,6,8-13C4]octanoate oxidation in intact rats was determined by nuclear magnetic resonance spectroscopy. We found that oxidation of 13C-octanoate in brain is avid and contributes approximately 20% to total brain oxidative energy production. Labeling patterns of glutamate and glutamine were distinct, and analysis of these metabolites indicated compartmentalized oxidation of octanoate in brain. Examination of liver and blood spectra revealed that label from 13C-octanoate was incorporated into glucose and ketones, which enabled calculation of its overall energy contribution to brain metabolism: glucose (predominantly unlabeled) and 13C-labeled octanoate can account for the entire oxidative metabolism of brain. Additionally, flux through anaplerotic pathways relative to tricarboxylic acid cycle flux (Y) was calculated to be 0.08 +/- 0.039 in brain, indicating that anaplerotic flux is significant and should be considered when assessing brain metabolism. Y was associated with the glutamine synthesis compartment, consistent with the view that anaplerotic flux occurs primarily in astrocytes.
Figures

References
-
- Auestad N, Korsak RA, Morrow JW, Edmond J ( 1991) Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem 56: 1376-1386. - PubMed
-
- Aureli T, di Cocco ME, Calvani M, Conti F ( 1997) The entry of [1- 13C]glucose into biochemical pathways reveals a complex compartmentation and metabolite trafficking between glia and neurons: a study by 13C-NMR spectroscopy. Brain Res 765: 218-227. - PubMed
-
- Brand A, Richter-Landsberg C, Leibfritz D ( 1997) Metabolism of acetate in rat brain neurons, astrocytes and cocultures: metabolic interactions between neurons and glia cells, monitored by NMR spectroscopy. Cell Mol Biol 43: 645-657. - PubMed
-
- Cerdan S, Künnecke B, Seelig J ( 1990) Cerebral metabolism of [1,2- 13C2]acetate as detected by in vivo and in vitro13C NMR. J Biol Chem 265: 12916-12926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
