Connexins are critical for normal myelination in the CNS
- PMID: 12843301
- PMCID: PMC6741267
- DOI: 10.1523/JNEUROSCI.23-13-05963.2003
Connexins are critical for normal myelination in the CNS
Abstract
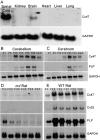
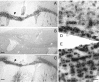
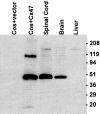
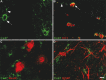
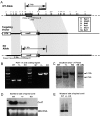
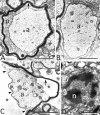
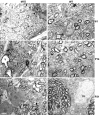
Mutations in Cx32, a gap-junction channel-forming protein, result in X-linked Charcot-Marie-Tooth disease, a demyelinating disease of the peripheral nervous system. However, although oligodendrocytes express Cx32, central myelination is unaffected. To explore this discrepancy, we searched for additional oligodendrocyte connexins. We found Cx47, which is expressed specifically in oligodendrocytes, regulated in parallel with myelin genes and partially colocalized with Cx32 in oligodendrocytes. Mice lacking either Cx47 or Cx32 are viable. However, animals lacking both connexins die by postnatal week 6 from profound abnormalities in central myelin, characterized by thin or absent myelin sheaths, vacuolation, enlarged periaxonal collars, oligodendrocyte cell death, and axonal loss. These data provide the first evidence that gap-junction communication is crucial for normal central myelination.
Figures


References
-
- Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH ( 1993) Connexin mutations in X-linked Charcot–Marie–Tooth disease. Science 262: 2039-2042. - PubMed
-
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM ( 1996) Expression of the APC tumor suppressor protein in oligodendroglia. Glia 17: 169-174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
