A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification
- PMID: 12843404
- PMCID: PMC166430
- DOI: 10.1073/pnas.1532257100
A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification
Abstract
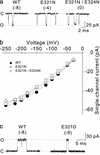
Large-conductance Ca2+-voltage-activated K+ channels (BK channels) control many key physiological processes, such as neurotransmitter release and muscle contraction. A signature feature of BK channels is that they have the largest single channel conductance of all K+ channels. Here we examine the mechanism of this large conductance. Comparison of the sequence of BK channels to lower-conductance K+ channels and to a crystallized bacterial K+ channel (MthK) revealed that BK channels have a ring of eight negatively charged glutamate residues at the entrance to the intracellular vestibule. This ring of charge, which is absent in lower-conductance K+ channels, is shown to double the conductance of BK channels for outward currents by increasing the concentration of K+ in the vestibule through an electrostatic mechanism. Removing the ring of charge converts BK channels to inwardly rectifying channels. Thus, a simple electrostatic mechanism contributes to the large conductance of BK channels.
Figures




References
-
- Cui, J. & Aldrich, R. W. (2000) Biochemistry 39, 15612-15619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

