Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres
- PMID: 12844504
- PMCID: PMC2343158
- DOI: 10.1113/jphysiol.2003.040022
Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres
Abstract
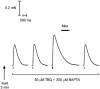
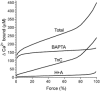
This study examined the relationship between the level of Ca2+ loading in the sarcoplasmic reticulum (SR) and the amount of Ca2+ released by an action potential (AP) in fast-twitch skeletal muscle fibres of the rat. Single muscle fibres were mechanically skinned and electric field stimulation was used to induce an AP in the transverse-tubular system and a resulting twitch response. Responses were elicited in the presence of known amounts (0-0.38 mM) of BAPTA, a fast Ca2+ buffer, with the SR Ca2+ pump either functional or blocked by 50 microM 2,5-di-tert-butyl-1,4-hydroquinone (TBQ). When Ca2+ reuptake was blocked, an estimate of the amount of Ca2+ released by an AP could be derived from the size of the force response. In a fibre with the SR loaded with Ca2+ at the endogenous level (approximately 1.2 mM, expressed as total Ca2+ per litre fibre volume; approximately one-third of maximal loading), a single AP triggered the release of approximately 230 microM Ca2+. If a second AP was elicited 10 ms after the first, only a further approximately 60 microM Ca2+ was released, the reduction probably being due to Ca2+ inactivation of Ca2+ release. When Ca2+ reuptake was blocked, APs applied 15 s apart elicited similar amounts of Ca2+ release (approximately 230 microM) on the first two or three occasions and then progressively less Ca2+ was released until the SR was fully depleted after a total of approximately eight APs. When the SR was loaded to near-maximal capacity (approximately 3-4 mM), each AP (or pair of APs 10 ms apart) still only released approximately the same amount of Ca2+ as that released when the fibre was endogenously loaded. Consistent with this, successive APs (15 s apart) elicited similar amounts of Ca2+ release approximately 10-16 times before the amount released declined, and the SR was fully depleted of Ca2+ after a total release calculated to be approximately 3-4 mM. When the SR was loaded maximally, increasing the [BAPTA] above 280 microM resulted in an increase in the amount of Ca2+ released per AP, probably because the greater level of cytoplasmic Ca2+ buffering prevented Ca2+ inactivation from adequately limiting Ca2+ release. These results show that the amount of Ca2+ released by AP stimulation in rat fast-twitch fibres normally stays virtually constant over a wide range of SR Ca2+ content, in spite of the likely large change in the electrochemical gradient for Ca2+. This was also found to be the case in toad twitch fibres. This constancy in Ca2+ release should help ensure precise regulation of force production in fast-twitch muscle in a range of circumstances.
Figures

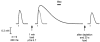


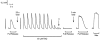


References
-
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. J Muscle Res Cell Motil. 1996;17:55–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

