Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum
- PMID: 12844505
- PMCID: PMC2343089
- DOI: 10.1113/jphysiol.2003.042176
Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum
Abstract
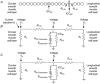
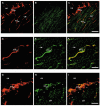
Intracellular recordings were made from short segments of the muscular wall of the guinea-pig gastric antrum. Preparations were impaled using two independent microelectrodes, one positioned in the circular layer and the other either in the longitudinal layer, in the network of myenteric interstitial cells of Cajal (ICCMY) or in the circular layer. Cells in each layer displayed characteristic patterns of rhythmical activity, with the largest signals being generated by ICCMY. Current pulses injected into the circular muscle layer produced electrotonic potentials in each cell layer, indicating that the layers are electrically interconnected. The amplitudes of these electrotonic potentials were largest in the circular layer and smallest in the longitudinal layer. An analysis of electrical coupling between the three layers suggests that although the cells in each layer are well coupled to neighbouring cells, the coupling between either muscle layer and the network of ICCMY is relatively poor. The electrical connections between ICCMY and the circular layer did not rectify. In parallel immunohistochemical studies, the distribution of the connexins Cx40, Cx43 and Cx45 within the antral wall was determined. Only Cx43 was detected; it was widely distributed on ICCMY and throughout the circular smooth muscle layer, being concentrated around ICCIM, but was less abundant in the circular muscle layer immediately adjacent to ICCMY. Although the electrophysiological studies indicate that smooth muscle cells in the longitudinal muscle layer are electrically coupled to each other, none of the connexins examined were detected in this layer.
Figures





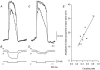
References
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit. Cell Tissue Res. 1997;290:11–20. - PubMed
-
- Coppen SR, Dupont E, Rothery S, Severs NJ. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ Res. 1998;82:232–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

