The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis
- PMID: 12847081
- PMCID: PMC2172718
- DOI: 10.1083/jcb.200212136
The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis
Abstract
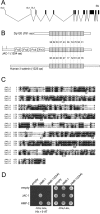
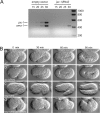
The cadherin-catenin complex is essential for tissue morphogenesis during animal development. In cultured mammalian cells, p120 catenin (p120ctn) is an important regulator of cadherin-catenin complex function. However, information on the role of p120ctn family members in cadherin-dependent events in vivo is limited. We have examined the role of the single Caenorhabditis elegans p120ctn homologue JAC-1 (juxtamembrane domain [JMD]-associated catenin) during epidermal morphogenesis. Similar to other p120ctn family members, JAC-1 binds the JMD of the classical cadherin HMR-1, and GFP-tagged JAC-1 localizes to adherens junctions in an HMR-1-dependent manner. Surprisingly, depleting JAC-1 expression using RNA interference (RNAi) does not result in any obvious defects in embryonic or postembryonic development. However, jac-1(RNAi) does increase the severity and penetrance of morphogenetic defects caused by a hypomorphic mutation in the hmp-1/alpha-catenin gene. In these hmp-1 mutants, jac-1 depletion causes failure of the embryo to elongate into a worm-like shape, a process that involves contraction of the epidermis. Associated with failed elongation is the detachment of actin bundles from epidermal adherens junctions and failure to maintain cadherin in adherens junctions. These results suggest that JAC-1 acts as a positive modulator of cadherin function in C. elegans.
Figures



References
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2000. The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113:1319–1334. - PubMed
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2001. Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13:604–610. - PubMed
-
- Chin-Sang, I.D., and A.D. Chisholm. 2000. Form of the worm: genetics of epidermal morphogenesis in C. elegans. Trends Genet. 16:544–551. - PubMed
-
- Costa, M., B.W. Draper, and J.R. Priess. 1997. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev. Biol. 184:373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

