Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae
- PMID: 12847085
- PMCID: PMC2172714
- DOI: 10.1083/jcb.200301022
Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae
Abstract
Rho1p, an essential Rho-type GTPase in Saccharomyces cerevisiae, activates its effectors in the GTP-bound form. Here, we show that Rho1p in secretory vesicles cannot activate 1,3-beta-glucan synthase, a cell wall synthesizing enzyme, during vesicular transport to the plasma membrane. Analyses with an antibody preferentially reacting with the GTP-bound form of Rho1p revealed that Rho1p remains in the inactive form in secretory vesicles. Rom2p, the GDP/GTP exchange factor of Rho1p, is preferentially localized on the plasma membrane even when vesicular transport is blocked. Overexpression of Rom2p results in delocalization of Rom2p and accumulation of 1,3-beta-glucan in secretory vesicles. Based on these results, we propose that Rho1p is kept inactive in intracellular secretory organelles, resulting in repression of the activity of the cell wall-synthesizing enzyme within cells.
Figures









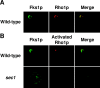


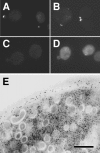


References
-
- Abe, M., I. Nishida, M. Minemura, H. Qadota, Y. Seyama, T. Watanabe, and Y. Ohya. 2001. Yeast 1,3-beta-glucan synthase activity is inhibited by phytosphingosine localized to the endoplasmic reticulum. J. Biol. Chem. 276:26923–26930. - PubMed
-
- Audhya, A., and S.D. Emr. 2002. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2:593–605. - PubMed
-
- Ayscough, K.R., J.J. Eby, T. Lila, H. Dewar, K.G. Kozminski, and D.G. Drubin. 1999. Sla1p is a functionally modular component of the yeast cortical actin cytoskeleton required for correct localization of both Rho1p-GTPase and Sla2p, a protein with talin homology. Mol. Biol. Cell. 10:1061–1075. - PMC - PubMed
-
- Cabib, E., D.H. Roh, M. Schmidt, L.B. Crotti, and A. Varma. 2001. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276:19679–19682. - PubMed
-
- Del Pozo, M.A., W.B. Kiosses, N.B. Alderson, N. Meller, K.M. Hahn, and M.A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

