Cross-presentation of disialoganglioside GD3 to natural killer T cells
- PMID: 12847141
- PMCID: PMC2196074
- DOI: 10.1084/jem.20030446
Cross-presentation of disialoganglioside GD3 to natural killer T cells
Abstract
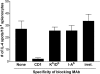
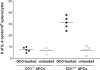
GD3, a ganglioside expressed on human melanoma, can be recognized by the humoral immune system. In this paper, we demonstrate that immunizing mice with the human melanoma cell line SK-MEL-28 (GD3+ GM2- CD1-) or with syngeneic APCs loaded with GD3 can induce a GD3-reactive natural killer T (NKT) cell response. GD3-reactive NKT cells were detected among splenocytes of immunized mice at frequencies of approximately 1:2000 both by ELISPOT and GD3-loaded mouse CD1d tetramer analysis. GD3-reactive NKT cells did not react with GM2, a closely related ganglioside, and were not detectable in unimmunized mice. GD3-reactive NKT cells initially produced IL-4 and IFN-gamma followed by IL-10. They were CD1d restricted in that reactivity was abrogated when APCs were blocked with anti-CD1d monoclonal antibody before being loaded with GD3 or when APCs from CD1d knockout mice were used. Because SK-MEL-28 does not express any isoform of human CD1, GD3 must be cross-presented by murine APCs in vivo. This is the first analysis of a natural ligand for mouse NKT cells and the first definitive paper of cross-presentation to NKT cells. This could be a mechanism for NKT cell recognition of tumor gangliosides in CD1- tumors.
Figures






Similar articles
-
Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand.Immunology. 2008 Jan;123(1):145-55. doi: 10.1111/j.1365-2567.2007.02760.x. Immunology. 2008. PMID: 18154620 Free PMC article.
-
Antibody response to GD3 ganglioside is independent of NKT cells.Cytotherapy. 2008;10(1):38-44. doi: 10.1080/14653240701762380. Cytotherapy. 2008. PMID: 18202973
-
The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner.Immunology. 2006 Sep;119(1):116-25. doi: 10.1111/j.1365-2567.2006.02413.x. Epub 2006 Jun 22. Immunology. 2006. PMID: 16792697 Free PMC article.
-
CD1d-mediated antigen presentation to natural killer T (NKT) cells.Crit Rev Immunol. 2003;23(5-6):403-19. doi: 10.1615/critrevimmunol.v23.i56.30. Crit Rev Immunol. 2003. PMID: 15030309 Review.
-
CD1-restricted T cells and tumor immunity.Curr Top Microbiol Immunol. 2007;314:293-323. doi: 10.1007/978-3-540-69511-0_12. Curr Top Microbiol Immunol. 2007. PMID: 17593666 Review.
Cited by
-
Mechanisms and Consequences of Antigen Presentation by CD1.Trends Immunol. 2016 Nov;37(11):738-754. doi: 10.1016/j.it.2016.08.011. Epub 2016 Sep 9. Trends Immunol. 2016. PMID: 27623113 Free PMC article. Review.
-
Targeting natural killer cells and natural killer T cells in cancer.Nat Rev Immunol. 2012 Mar 22;12(4):239-52. doi: 10.1038/nri3174. Nat Rev Immunol. 2012. PMID: 22437937 Free PMC article. Review.
-
Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity.J Exp Med. 2005 Dec 5;202(11):1517-26. doi: 10.1084/jem.20051625. Epub 2005 Nov 28. J Exp Med. 2005. PMID: 16314439 Free PMC article.
-
Tissue-Resident Innate Immune Cell-Based Therapy: A Cornerstone of Immunotherapy Strategies for Cancer Treatment.Front Cell Dev Biol. 2022 May 26;10:907572. doi: 10.3389/fcell.2022.907572. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35757002 Free PMC article. Review.
-
The Role of NKT Cells in Glioblastoma.Cells. 2021 Jun 30;10(7):1641. doi: 10.3390/cells10071641. Cells. 2021. PMID: 34208864 Free PMC article. Review.
References
-
- Porcelli, S.A., and R.L. Modlin. 1999. The CD1 System: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297–329. - PubMed
-
- Moody, D.B., B.B. Reinhold, M.R. Guy, E.M. Beckman, D.E. Frederique, S.T. Furlong, S. Ye, V.N. Reinhold, P.A. Sieling, R.L. Modlin, G.S. Besra, and S.A. Porcelli. 1997. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 278:283–286. - PubMed
-
- Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Taniguchi. 1997. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 278:1626–1629. - PubMed
-
- Shamshiev, A., A. Donda, T.I. Prigozy, L. Mori, V. Chigorno, C.A. Benedict, L. Kappos, S. Sonnino, M. Kronenberg, and G. De Libero. 2000. The αβ T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 13:255–264. - PubMed
-
- Porcelli, S.A., B.W. Segelke, M. Sugita, I.A. Wilson, and M.B. Brenner. 1998. The CD1 family of lipid antigen-presenting molecules. Immunol. Today. 19:362–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

