Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans
- PMID: 12847205
- PMCID: PMC2343308
- DOI: 10.1113/jphysiol.2003.042135
Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans
Abstract
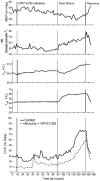
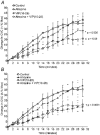
Active vasodilatation (AVD) in human, non-glabrous skin depends on functional cholinergic fibres but not on acetylcholine (ACh). We tested whether AVD is a redundant system in which ACh and vasoactive intestinal polypeptide (VIP) are co-released from cholinergic nerves. (1) We administered VIP by intradermal microdialysis to four discrete areas of skin in the presence of different levels of the VIP receptor antagonist, VIP(10-28), also delivered by microdialysis. Skin blood flow (SkBF) was continuously monitored by laser Doppler flowmetry (LDF). Mean arterial pressure (MAP) was measured non-invasively and cutaneous vascular conductance (CVC) calculated as LDF/MAP. Subjects were supine and wore water-perfused suits to control whole-body skin temperature (Tsk) at 34 degrees C. Concentrations of 54 microM, 107 microM, or 214 microM VIP(10-28) were perfused via intradermal microdialysis at 2 microl min-1 for approximately 1 h. Then 7.5 microM VIP was added to the perfusate containing VIP(10-28) at the three concentrations or Ringer solution and perfusion was continued for 45-60 min. At the control site, this level of VIP caused approximately the vasodilatation typical of heat stress. All VIP(10-28)-treated sites displayed an attenuated dilatation in response to the VIP. The greatest attenuation was observed at the site that received 214 microM VIP(10-28) (P < 0.01). (2) We used 214 microM VIP(10-28) alone and with the iontophoretically administered muscarinic receptor antagonist atropine (400 microA cm-2, 45 s, 10 mM) in heated subjects to test the roles of VIP and ACh in AVD. Ringer solution and 214 microM VIP(10-28) were each perfused at two sites, one of which in each case was pretreated with atropine. After 1 h of VIP(10-28) treatment, individuals underwent 45-60 min of whole-body heating (Tsk to 38.5 degrees C). VIP(10-28), alone or in combination with atropine, attenuated the increase in CVC during heat stress, suggesting an important role for VIP in AVD.
Figures


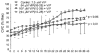

References
-
- Aggestrup S, Uddman R, Sundler F, Fahrenkrug J, Hakanson R, Sorensen HR, Hambraeus G. Lack of vasoactive intestinal polypeptide nerves in esophageal achalasia. Gastroenterol. 1983;84:924–927. - PubMed
-
- Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Investig Dermatol. 1994;102:807–811. - PubMed
-
- Chakder S, Rattan S. The entire vasoactive intestinal polypeptide molecule is required for the activation of the vasoactive intestinal polypeptide receptor: functional and binding studies on opossum internal anal sphincter smooth muscle. J Pharmacol Exp Ther. 1993;266:392–399. - PubMed
-
- Eedy DJ, Shaw C, Armstrong EP, Johnston CF, Buchanan KD. Vasoactive intestinal peptide (VIP) and peptide histidine methionine (PHM) in human eccrine sweat glands: demonstration of innervation, specific binding sites and presence in secretions. Br J Dermatol. 1990;123:65–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

