Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties
- PMID: 12847206
- PMCID: PMC2343292
- DOI: 10.1113/jphysiol.2003.046417
Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties
Abstract
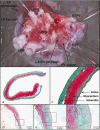
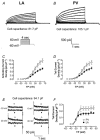
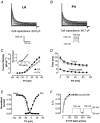
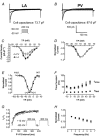
Pulmonary vein (PV) cardiomyocytes play an important role in atrial fibrillation; however, little is known about their specific cellular electrophysiological properties. We applied standard microelectrode recording and whole-cell patch-clamp to evaluate action potentials and ionic currents in canine PVs and left atrium (LA) free wall. Resting membrane potential (RMP) averaged -66 +/- 1 mV in PVs and -74 +/- 1 mV in LA (P < 0.0001) and action potential amplitude averaged 76 +/- 2 mV in PVs vs. 95 +/- 2 mV in LA (P < 0.0001). PVs had smaller maximum phase 0 upstroke velocity (Vmax: 98 +/- 9 vs. 259 +/- 16 V s(-1), P < 0.0001) and action potential duration (APD): e.g. at 2 Hz, APD to 90% repolarization in PVs was 84 % of LA (P < 0.05). Na+ current density under voltage-clamp conditions was similar in PV and LA, suggesting that smaller Vmax in PVs was due to reduced RMP. Inward rectifier current density in the PV cardiomyocytes was approximately 58% that in the LA, potentially accounting for the less negative RMP in PVs. Slow and rapid delayed rectifier currents were greater in the PV (by approximately 60 and approximately 50 %, respectively), whereas transient outward K+ current and L-type Ca2+ current were significantly smaller (by approximately 25 and approximately 30%, respectively). Na(+)-Ca(2+)-exchange (NCX) current and T-type Ca2+ current were not significantly different. In conclusion, PV cardiomyocytes have a discrete distribution of transmembrane ion currents associated with specific action potential properties, with potential implications for understanding PV electrical activity in cardiac arrhythmias.
Figures

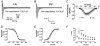
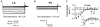

References
-
- Allessie MA, Boyden PA, Camm AJ, Kleber AG, Lab MJ, Legato MJ, Rosen MR, Schwartz PJ, Spooner PM, Van Wagoner DR, Waldo AL. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. - PubMed
-
- Barber MJ, Starmer CF, Grant AO. Blockade of cardiac sodium channels by amitriptyline and diphenylhydantoin. Evidence for two use-dependent binding sites. Circ Res. 1991;69:677–696. - PubMed
-
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death. The Framingham heart study. Circulation. 1998;98:946–952. - PubMed
-
- Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, Nattel S. Effects of chromanol 293B, a selective blocker of the slow component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc Res. 1998;38:441–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

