Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases
- PMID: 12847291
- PMCID: PMC166400
- DOI: 10.1073/pnas.1533136100
Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases
Abstract
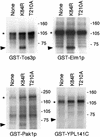
The Snf1/AMP-activated protein kinase (AMPK) family plays fundamental roles in cellular responses to metabolic stress in eukaryotes. In humans, AMPK regulates lipid and glucose metabolism and has been implicated in such metabolic disorders as diabetes and obesity and in cardiac abnormalities. Snf1 and AMPK are the downstream components of kinase cascades, but the upstream kinase(s) have remained elusive. We have here identified three yeast kinases, Pak1p, Tos3p, and Elm1p, that activate Snf1 kinase in vivo. Triple deletion of the cognate genes causes a Snf- mutant phenotype and abolishes Snf1 catalytic activity. All three kinases phosphorylate recombinant Snf1p on the activation-loop threonine. Moreover, Tos3p phosphorylates mammalian AMPK on the equivalent residue and activates the enzyme, suggesting functional conservation of the upstream kinases between yeast and mammals. We further show that the closely related mammalian LKB1 kinase, which is associated with Peutz-Jeghers cancer-susceptibility syndrome, phosphorylates and activates AMPK in vitro. Thus, the identification of the yeast upstream kinases should facilitate identification of the corresponding, physiologically important mammalian upstream kinases.
Figures



References
-
- Hardie, D. G., Carling, D. & Carlson, M. (1998) Annu. Rev. Biochem. 67, 821–855. - PubMed
-
- Kemp, B. E., Stapleton, D., Campbell, D. J., Chen, Z. P., Murthy, S., Walter, M., Gupta, A., Adams, J. J., Katsis, F., Van Denderen, B., et al. (2003) Biochem. Soc. Trans. 31, 162–168. - PubMed
-
- Minokoshi, Y., Kim, Y. B., Peroni, O. D., Fryer, L. G., Muller, C., Carling, D. & Kahn, B. B. (2002) Nature 415, 339–343. - PubMed
-
- Mu, J., Brozinick, J. T. J., Valladares, O., Bucan, M. & Birnbaum, M. J. (2001) Mol. Cell 7, 1085–1094. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

