Lack of pharmacokinetic interaction between retigabine and phenobarbitone at steady-state in healthy subjects
- PMID: 12848774
- PMCID: PMC1884339
- DOI: 10.1046/j.1365-2125.2003.01825.x
Lack of pharmacokinetic interaction between retigabine and phenobarbitone at steady-state in healthy subjects
Abstract
Aims: To evaluate potential pharmacokinetic interactions between phenobarbitone and retigabine, a new antiepileptic drug.
Methods: Fifteen healthy men received 200 mg of retigabine on day 1. On days 4-32, phenobarbitone 90 mg was administered at 22.00 h. On days 26-32, increasing doses of retigabine were given to achieve a final dose of 200 mg every 8 h on day 32. The pharmacokinetics of retigabine were determined on days 1 and 32, and those for phenobarbitone on days 25 and 31.
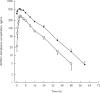
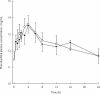
Results: After administration of a single 200 mg dose, retigabine was rapidly absorbed and eliminated with a mean terminal half-life of 6.7 h, a mean AUC of 3936 ng x ml(-1) x h and a mean apparent clearance of 0.76 l x h(-1) x kg(-1). Similar exposure to the partially active acetylated metabolite (AWD21-360) of retigabine was observed. After administration of phenobarbitone dosed to steady-state, the pharmacokinetics of retigabine at steady-state were similar (AUC of 4433 ng x ml(-1) x h and t1/2 of 8.5 h) to those of retigabine alone. The AUC of phenobarbitone was 298 mg x l(-1) x h when administered alone and 311 mg x ml(-1) x h after retigabine administration. The geometric mean ratios and 90% confidence intervals of the AUC were 1.11 (0.97, 1.28) for retigabine, 1.01 (0.88, 1.06) for AWD21-360 and 1.04 (0.96, 1.11) for phenobarbitone. Individual and combined treatments were generally well tolerated. One subject was withdrawn from the study on day 10 due to severe abdominal pain. Headache was the most commonly reported adverse event. No clinically relevant changes were observed in the electrocardiograms, vital signs or laboratory measurements.
Conclusions: There was no pharmacokinetic interaction between retigabine and phenobarbitone in healthy subjects. No dosage adjustment is likely to be necessary when retigabine and phenobarbitone are coadministered to patients.
Figures

References
-
- Rundfeldt CNR. Investigations into the mechanism of action of the new anticonvulsant retigabine. Arzneimittel Forsch Drug Res. 2000;50:1063–1070. - PubMed
-
- Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600. - PubMed
-
- Rostock A, Tober C, Rundfeldt C, et al. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23:211–223. - PubMed
-
- Armand V, Rundfeldt C, Heinemann U. Effects of retigabine (D-23129) on different patterns of epileptiform activity induced by low magnesium in rat entorhinal cortex hippocampal slices. Epilepsia. 2000;41:28–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

