Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression
- PMID: 12848900
- PMCID: PMC179893
- DOI: 10.1186/1472-6793-3-5
Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression
Abstract
Background: Fasting and diabetes are characterized by elevated glucocorticoids and reduced insulin, leptin, elevated hypothalamic AGRP and NPY mRNA, and reduced hypothalamic POMC mRNA. Although leptin replacement can reverse changes in hypothalamic gene expression associated with fasting and diabetes, leptin also normalizes corticosterone; therefore the extent to which the elevated corticosterone contributes to the regulation of hypothalamic gene expression in fasting and diabetes remains unclear. To address if elevated corticosterone is necessary for hypothalamic responses to fasting and diabetes, we assessed the effects of adrenalectomy on hypothalamic gene expression in 48-hour-fasted or diabetic mice. To assess if elevated corticosterone is sufficient for the hypothalamic responses to fasting and diabetes, we assessed the effect of corticosterone pellets implanted for 48 hours on hypothalamic gene expression.
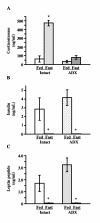
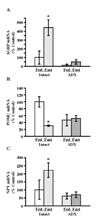
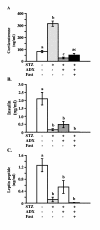
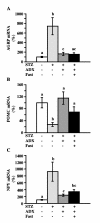
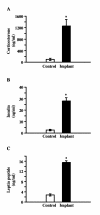
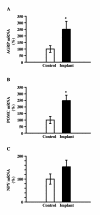
Results: Fasting and streptozotocin-induced diabetes elevated plasma glucocorticoid levels and reduced serum insulin and leptin levels. Adrenalectomy prevented the rise in plasma glucocorticoids associated with fasting and diabetes, but not the associated reductions in insulin or leptin. Adrenalectomy blocked the effects of fasting and diabetes on hypothalamic AGRP, NPY, and POMC expression. Conversely, corticosterone implants induced both AGRP and POMC mRNA (with a non-significant trend toward induction of NPY mRNA), accompanied by elevated insulin and leptin (with no change in food intake or body weight).
Conclusion: These data suggest that elevated plasma corticosterone mediate some effects of fasting and diabetes on hypothalamic gene expression. Specifically, elevated plasma corticosterone is necessary for the induction of NPY mRNA with fasting and diabetes; since corticosterone implants only produced a non-significant trend in NPY mRNA, it remains uncertain if a rise in corticosterone may be sufficient to induce NPY mRNA. A rise in corticosterone is necessary to reduce hypothalamic POMC mRNA with fasting and diabetes, but not sufficient for the reduction of hypothalamic POMC mRNA. Finally, elevated plasma corticosterone is both necessary and sufficient for the induction of hypothalamic AGRP mRNA with fasting and diabetes.
Figures
Similar articles
-
Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats.Brain Res. 2002 Dec 20;958(1):130-8. doi: 10.1016/s0006-8993(02)03674-0. Brain Res. 2002. PMID: 12468037
-
Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin.Endocrinology. 1999 Oct;140(10):4551-7. doi: 10.1210/endo.140.10.6966. Endocrinology. 1999. PMID: 10499510
-
Unlike leptin, ciliary neurotrophic factor does not reverse the starvation-induced changes of serum corticosterone and hypothalamic neuropeptide levels but induces expression of hypothalamic inhibitors of leptin signaling.Diabetes. 2000 Nov;49(11):1890-6. doi: 10.2337/diabetes.49.11.1890. Diabetes. 2000. PMID: 11078456
-
Neuroendocrine actions and regulation of hypothalamic neuropeptide Y during lactation.Peptides. 2007 Feb;28(2):447-52. doi: 10.1016/j.peptides.2006.09.025. Epub 2007 Jan 22. Peptides. 2007. PMID: 17241697 Free PMC article. Review.
-
CNS-periphery relationships and body weight homeostasis: influence of the glucocorticoid status.Int J Obes Relat Metab Disord. 2000 Jun;24 Suppl 2:S74-6. doi: 10.1038/sj.ijo.0801283. Int J Obes Relat Metab Disord. 2000. PMID: 10997614 Review.
Cited by
-
Control of energy balance by hypothalamic gene circuitry involving two nuclear receptors, neuron-derived orphan receptor 1 and glucocorticoid receptor.Mol Cell Biol. 2013 Oct;33(19):3826-34. doi: 10.1128/MCB.00385-13. Epub 2013 Jul 29. Mol Cell Biol. 2013. PMID: 23897430 Free PMC article.
-
Neuroendocrinology of reward in anorexia nervosa and bulimia nervosa: Beyond leptin and ghrelin.Mol Cell Endocrinol. 2019 Nov 1;497:110320. doi: 10.1016/j.mce.2018.10.018. Epub 2018 Nov 2. Mol Cell Endocrinol. 2019. PMID: 30395874 Free PMC article. Review.
-
Effects of lifestyle factors on leukocytes in cardiovascular health and disease.Nat Rev Cardiol. 2024 Mar;21(3):157-169. doi: 10.1038/s41569-023-00931-w. Epub 2023 Sep 26. Nat Rev Cardiol. 2024. PMID: 37752350 Review.
-
Negative energy balance hinders prosocial helping behavior.Proc Natl Acad Sci U S A. 2023 Apr 11;120(15):e2218142120. doi: 10.1073/pnas.2218142120. Epub 2023 Apr 6. Proc Natl Acad Sci U S A. 2023. PMID: 37023123 Free PMC article.
-
Brain-specific homeobox factor as a target selector for glucocorticoid receptor in energy balance.Mol Cell Biol. 2013 Jul;33(14):2650-8. doi: 10.1128/MCB.00094-13. Epub 2013 May 13. Mol Cell Biol. 2013. PMID: 23671185 Free PMC article.
References
-
- Mobbs C, Mizuno T. Leptin regulation of proopiomelanocortin. Front Horm Res. 2000;26:57–70. - PubMed
-
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. - PubMed
-
- Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140:4551–4557. doi: 10.1210/en.140.10.4551. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

