Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy
- PMID: 12851463
- PMCID: PMC166412
- DOI: 10.1073/pnas.1537554100
Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy
Abstract
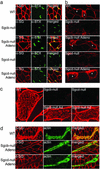
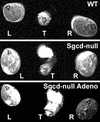
Limb-girdle muscular dystrophy types 2E and F are characterized by skeletal muscle weakness and often cardiomyopathy and are due to mutations in the genes encoding beta- and delta-sarcoglycan. We previously demonstrated that loss of sarcoglycans in smooth muscle leads to constrictions of the microvasculature that contributes to the cardiac phenotype. It is unclear how vasculature abnormalities affect skeletal muscle. We injected recombinant beta- or delta-sarcoglycan adenoviruses into skeletal muscles of corresponding null mice. We hypothesized that the adenoviruses would not transduce vascular smooth muscle, and we would only target skeletal muscle. Indeed, sustained expression of intact sarcoglycan-sarcospan complex was noted at the sarcolemma, neuromuscular junction, myotendinous junction, and in peripheral nerve, but not in vascular smooth muscle. Gene transfer of the corresponding deleted sarcoglycan gene preserved sarcolemmal integrity, prevented pathological dystrophy and hypertrophy, and protected against exercised-induced damage. We conclude that vascular dysfunction is not a primary cause of beta- and delta-sarcoglycan-deficient muscular dystrophy. In addition, we show successful functional rescue of entire muscles after adenovirus-mediated gene delivery. Thus, virus-mediated gene transfer of sarcoglycans to skeletal muscle in combination with pharmacological prevention of cardiomyopathy constitute promising therapeutic strategies for limb-girdle muscular dystrophies.
Figures


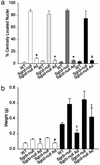
 , quadriceps
, quadriceps
 , and calf (▪)
muscles. *, Difference from Sgcd-null, P ≈ 0; #, difference
from Sgcd-null, P < 0.0015. (b) Tibialis anterior (□)
and hamstring (▪) muscle masses of 30-wk-old mice. *, Difference
from Sgcb-null, P < 0.026; #, difference from Sgcd-null,
P < 0.0002; †, difference from Sgcb-null and WT, P
< 0.001 and P < 0.008, respectively; ‡, difference from
Sgcd-null, P < 0.027. Statistical significance was examined by
using Student's t test.
, and calf (▪)
muscles. *, Difference from Sgcd-null, P ≈ 0; #, difference
from Sgcd-null, P < 0.0015. (b) Tibialis anterior (□)
and hamstring (▪) muscle masses of 30-wk-old mice. *, Difference
from Sgcb-null, P < 0.026; #, difference from Sgcd-null,
P < 0.0002; †, difference from Sgcb-null and WT, P
< 0.001 and P < 0.008, respectively; ‡, difference from
Sgcd-null, P < 0.027. Statistical significance was examined by
using Student's t test.References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

