Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development
- PMID: 12852851
- PMCID: PMC3918747
- DOI: 10.1016/s1534-5807(03)00169-2
Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development
Abstract
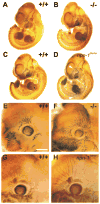
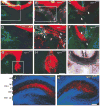
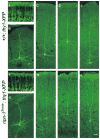
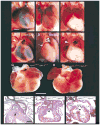
Neuropilin-1 (Npn-1) is a receptor that binds multiple ligands from structurally distinct families, including secreted semaphorins (Sema) and vascular endothelial growth factors (VEGF). We generated npn-1 knockin mice, which express an altered ligand binding site variant of Npn-1, and npn-1 conditional null mice to establish the cell-type- and ligand specificity of Npn-1 function in the developing cardiovascular and nervous systems. Our results show that VEGF-Npn-1 signaling in endothelial cells is required for angiogenesis. In striking contrast, Sema-Npn-1 signaling is not essential for general vascular development but is required for axonal pathfinding by several populations of neurons in the CNS and PNS. Remarkably, both Sema-Npn-1 signaling and VEGF-Npn-1 signaling are critical for heart development. Therefore, Npn-1 is a multifunctional receptor that mediates the activities of structurally distinct ligands during development of the heart, vasculature, and nervous system.
Figures



Similar articles
-
Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165.J Biol Chem. 2002 May 17;277(20):18069-76. doi: 10.1074/jbc.M201681200. Epub 2002 Mar 8. J Biol Chem. 2002. PMID: 11886873
-
Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121).J Biol Chem. 2001 Jul 6;276(27):25520-31. doi: 10.1074/jbc.M102315200. Epub 2001 May 1. J Biol Chem. 2001. PMID: 11333271
-
Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A.Blood. 2006 May 15;107(10):3892-901. doi: 10.1182/blood-2005-10-4113. Epub 2006 Jan 19. Blood. 2006. PMID: 16424390 Free PMC article.
-
Neuropilins in the Context of Tumor Vasculature.Int J Mol Sci. 2019 Feb 1;20(3):639. doi: 10.3390/ijms20030639. Int J Mol Sci. 2019. PMID: 30717262 Free PMC article. Review.
-
Functions of semaphorins in axon guidance and neuronal regeneration.Jpn J Pharmacol. 2000 Apr;82(4):273-9. doi: 10.1254/jjp.82.273. Jpn J Pharmacol. 2000. PMID: 10875745 Review.
Cited by
-
Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation.Circ Res. 2012 Aug 3;111(4):437-45. doi: 10.1161/CIRCRESAHA.112.269316. Epub 2012 Jun 21. Circ Res. 2012. PMID: 22723296 Free PMC article.
-
A balance of noncanonical Semaphorin signaling from the cerebrospinal fluid regulates apical cell dynamics during corticogenesis.Sci Adv. 2022 Nov 16;8(46):eabo4552. doi: 10.1126/sciadv.abo4552. Epub 2022 Nov 18. Sci Adv. 2022. PMID: 36399562 Free PMC article.
-
Getting neural circuits into shape with semaphorins.Nat Rev Neurosci. 2012 Sep;13(9):605-18. doi: 10.1038/nrn3302. Epub 2012 Aug 16. Nat Rev Neurosci. 2012. PMID: 22895477 Review.
-
Semaphorin 6A regulates angiogenesis by modulating VEGF signaling.Blood. 2012 Nov 8;120(19):4104-15. doi: 10.1182/blood-2012-02-410076. Epub 2012 Sep 24. Blood. 2012. PMID: 23007403 Free PMC article.
-
NRP1 and NRP2 cooperate to regulate gangliogenesis, axon guidance and target innervation in the sympathetic nervous system.Dev Biol. 2012 Sep 15;369(2):277-85. doi: 10.1016/j.ydbio.2012.06.026. Epub 2012 Jul 10. Dev Biol. 2012. PMID: 22790009 Free PMC article.
References
-
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones, and heart. Nature. 1996;383:525–528. - PubMed
-
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. - PubMed
-
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. - PubMed
-
- Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. - PubMed
-
- Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chedotal A, Tessier-Lavigne M. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

