Recombinant single-chain variable fragment antibodies directed against Clostridium difficile toxin B produced by use of an optimized phage display system
- PMID: 12853390
- PMCID: PMC164272
- DOI: 10.1128/cdli.10.4.587-595.2003
Recombinant single-chain variable fragment antibodies directed against Clostridium difficile toxin B produced by use of an optimized phage display system
Abstract
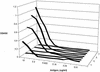
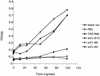
Recombinant antibody cloning and phage display technologies were used to produce single-chain antibodies (scFv) against Clostridium difficile toxin B. The starting material was the mouse B cell hybridoma line 5A8, which generates a monoclonal antibody against the toxin. The integrated cloning, screening, and phage display system of Krebber et al. (J. Immunol. Methods 201:35-55, 1997) allowed us to rapidly obtain toxin B-binding scFv sequences derived from the hybridoma cell line. The best candidate scFv sequences, based on preliminary enzyme-linked immunosorbent assay (ELISA) screening data were then subcloned into the compatible expression vector. Recombinant single-chain antibodies were expressed in Escherichia coli. A 29-kDa band was observed on polyacrylamide gel electrophoresis as predicted. The expressed product was characterized by immunoblotting and detection with an anti-FLAG antibody. The toxin B-binding function of the single-chain antibody was shown by a sandwich ELISA. The antibody was highly specific for toxin B and did not cross-react with material isolated from a toxin B-negative C. difficile strain. The sensitivity of the soluble single-chain antibody is significantly higher than the original monoclonal antibody based on ELISA data and could detect a minimum of 10 ng of toxin B/well. Competitive ELISAs established that the affinity of the 5A8 parent antibody and the best representative (clone 10) of the single-chain antibodies were similar and in the range of 10(-8) M. We propose that recombinant antibody technology is a rapid and effective approach to the development of the next generation of immunodiagnostic reagents.
Figures




References
-
- Anand, A., B. Bashey, T. Mir, and A. E. Glatt. 1994. Epidemiology, clinical manifestations, and outcome of Clostridium difficile-associated diarrhea. Am. J. Gastroenterol. 89:519-523. - PubMed
-
- Arndt, K., K. M. Muller, and A. Pluckthun. 1998. Factors influencing the dimer to monomer transition of an antibody single-chain Fv fragment. Biochemistry 37:12918-12926. - PubMed
-
- Bartlett, J. G., A B. Onderdonk, and R. L. Cinseros. 1977. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J. Infect. Dis. 136:701-705. - PubMed
-
- Bartlett, J. G., T. W. Chang, M. Gurwith, S. L. Gorbach, and A. B. Onderdonk. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 298:531-534. - PubMed
-
- Berger, S. L., and J. M. Chirgwin. 1989. Isolation of RNA. Methods Enzymol. 180:3-13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

