T-cell immune response assessment as a complement to serology and intranasal protection assays in determining the protective immunity induced by acellular pertussis vaccines in mice
- PMID: 12853397
- PMCID: PMC164277
- DOI: 10.1128/cdli.10.4.637-642.2003
T-cell immune response assessment as a complement to serology and intranasal protection assays in determining the protective immunity induced by acellular pertussis vaccines in mice
Abstract
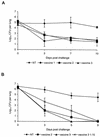
The relative value of antibodies and/or T-cell immune responses to Bordetella pertussis antigens in the immunity induced by acellular pertussis (aP) vaccines is still an open issue, probably due to the incomplete knowledge on the mechanisms of protective immunity to pertussis. The relevance of T-cell immune responses in protection from pertussis has been demonstrated in murine and human models of infection; thus, in this study, the ability of different vaccine preparations of three component (pertussis toxin, filamentous hemagglutinin, and pertactin) aP vaccines to induce T-cell responses was investigated in mice. All vaccine preparations examined passed the immunogenicity control test, based on antibody titer assessment, according to European Pharmacopoeia standards, and protected mice from B. pertussis intranasal challenge, but not all preparations were able to prime T cells to pertussis toxin, the specific B. pertussis antigen. In particular, one vaccine preparation was unable to induce proliferation and gamma interferon (IFN-gamma) production while the other two gave borderline results. The evaluation of T-cell responses to pertussis toxin antigen may provide information on the protective immunity induced by aP vaccines in animal models. Considering the critical role of the axis interleukin-12-IFN-gamma for protection from pertussis, our results suggest that testing the induction of a key protective cytokine such as IFN-gamma could be an additional tool for the evaluation of the immune response induced by aP vaccines.
Figures
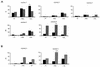
Similar articles
-
Evaluation of efficacy in terms of antibody levels and cell-mediated immunity of acellular pertussis vaccines in a murine model of respiratory infection.FEMS Immunol Med Microbiol. 2002 Jul 12;33(3):219-25. doi: 10.1111/j.1574-695X.2002.tb00594.x. FEMS Immunol Med Microbiol. 2002. PMID: 12110485
-
Intranasal application of a bifunctional pertactin-RTX fusion antigen elicits protection of mouse airway mucosa against Bordetella pertussis colonization.mSphere. 2025 Apr 29;10(4):e0095924. doi: 10.1128/msphere.00959-24. Epub 2025 Mar 31. mSphere. 2025. PMID: 40162794 Free PMC article.
-
Cellular immunity in adolescents and adults following acellular pertussis vaccine administration.Clin Vaccine Immunol. 2007 Mar;14(3):288-92. doi: 10.1128/CVI.00364-06. Epub 2007 Jan 31. Clin Vaccine Immunol. 2007. PMID: 17267589 Free PMC article.
-
T-cell immune responses to Bordetella pertussis infection and vaccination.Pathog Dis. 2015 Oct;73(7):ftv051. doi: 10.1093/femspd/ftv051. Epub 2015 Aug 4. Pathog Dis. 2015. PMID: 26242279 Review.
-
Pertussis: current concepts of pathogenesis and prevention.Pediatr Infect Dis J. 1997 Apr;16(4 Suppl):S78-84. doi: 10.1097/00006454-199704001-00002. Pediatr Infect Dis J. 1997. PMID: 9109161 Review.
Cited by
-
Vaccine-Induced Cellular Immunity against Bordetella pertussis: Harnessing Lessons from Animal and Human Studies to Improve Design and Testing of Novel Pertussis Vaccines.Vaccines (Basel). 2021 Aug 7;9(8):877. doi: 10.3390/vaccines9080877. Vaccines (Basel). 2021. PMID: 34452002 Free PMC article. Review.
-
Production and characterization of recombinant pertactin, fimbriae 2 and fimbriae 3 from Bordetella pertussis.BMC Microbiol. 2009 Dec 29;9:274. doi: 10.1186/1471-2180-9-274. BMC Microbiol. 2009. PMID: 20040101 Free PMC article.
-
International Bordetella pertussis assay standardization and harmonization meeting report. Centers for Disease Control and Prevention, Atlanta, Georgia, United States, 19-20 July 2007.Vaccine. 2009 Feb 5;27(6):803-14. doi: 10.1016/j.vaccine.2008.11.072. Epub 2008 Dec 9. Vaccine. 2009. PMID: 19071179 Free PMC article.
-
Immunogenicity of a whole-cell pertussis vaccine with low lipopolysaccharide content in infants.Clin Vaccine Immunol. 2009 Apr;16(4):544-50. doi: 10.1128/CVI.00339-08. Epub 2009 Mar 4. Clin Vaccine Immunol. 2009. PMID: 19261771 Free PMC article. Clinical Trial.
-
Delayed BCG vaccination results in minimal alterations in T cell immunogenicity of acellular pertussis and tetanus immunizations in HIV-exposed infants.Vaccine. 2015 Sep 11;33(38):4782-9. doi: 10.1016/j.vaccine.2015.07.096. Epub 2015 Aug 7. Vaccine. 2015. PMID: 26259542 Free PMC article. Clinical Trial.
References
-
- Ad Hoc Group for the Study of Pertussis Vaccines. 1988. Placebo-controlled trial of two acellular pertussis vaccines in Sweden: protective efficacy and adverse events. Lancet i:955-960. - PubMed
-
- Ausiello, C. M., P. Sestili, C. Locardi, M. Logozzi, P. Rizza, E. Parlanti, L. Yang, A. Modica, A. Modesti, P. Musiani, and F. Belardelli. 1994. Defective response to T cell mitogens in mice injected with human immunodeficiency virus type 1-infected U937 cells. J. Gen. Virol. 75:2789-2794. - PubMed
-
- Ausiello, C. M., R. Lande, A. la Sala, F. Urbani, and A. Cassone. 1998. Cell-mediated immune response of healthy adults to Bordetella pertussis vaccine antigens. J. Infect. Dis. 178:466-470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

