Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and muller cells
- PMID: 12853421
- PMCID: PMC6740352
- DOI: 10.1523/JNEUROSCI.23-14-06030.2003
Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and muller cells
Abstract
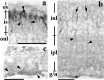
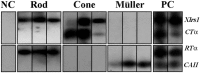
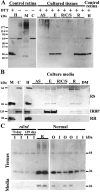
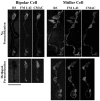
Usually, photoreceptors interact with other retinal cells through the neurotransmitter glutamate. Here we describe a nonsynaptic interaction via a secreted protein, retinoschisin. Using in situ hybridization, we found that from early postnatal life retinoschisin mRNA is present only in the outer retina of the mouse, and with single-cell RT-PCR we demonstrated its localization in rod and cone photoreceptor cells but not in Müller cells. Western blot analyses of proteins from cultured ocular tissues and microdissected outer and inner retinas, as well as from the culture media of these samples, showed that retinoschisin is secreted from the photoreceptor cells. Immunostaining of permeabilized and nonpermeabilized dissociated retinal cells revealed that retinoschisin is mainly inside and outside the photoreceptors, outside bipolar cells, and associated with plasma membranes of Müller cells and inside their distal processes. Because we showed previously that retinoschisin is distributed all over the retina, our current data suggest that after synthesis and secretion by the photoreceptors, retinoschisin reaches the surface of retinal cells and mediates interactions/adhesion between photoreceptor, bipolar, and Müller cells, contributing to the maintenance of the cytoarchitectural integrity of the retina. These interactions may not occur when the gene encoding retinoschisin is mutated, as it occurs in X-linked juvenile retinoschisis, a disease that results in morphological and electrophysiological defects of the retina.
Figures



References
-
- Alexander KR, Fishman GA, Grover S ( 2000) Temporal frequency deficits in the electroretinogram of the cone system in X-linked retinoschisis. Vision Res 40: 2861–2868. - PubMed
-
- Ando A, Takahashi K, Sho K, Matsushima M, Okamura A, Uyama M ( 2000) Histopathological findings of X-linked retinoschisis with neovascular glaucoma. Graefe's Arch Clin Exp Ophthalmol 238: 1–7. - PubMed
-
- Apweiler R, Attwood TK, Bairoch A, Bateman A, Birney E, Biswas M, Bucher P, Cerutti L, Corpet F, Croning MDR, Durbin R, Falquet L, Fleischmann W, Gouzy J, Hermjakob H, Hulo N, Jonassen I, Kahn D, Kanapin A, Karavidopoulou Y, et al. ( 2001) The InterPro database, an integrated documentation resource for protein families, domains and functional sites. Nucleic Acids Res 29: 37–40. - PMC - PubMed
-
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ ( 1996) A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87: 1059–1068. - PubMed
-
- Blanks JC, Adinolfi AM, Lolley RN ( 1974) Synaptogenesis in the photoreceptor terminal of the mouse retina. J Comp Neurol 156: 81–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
