Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse
- PMID: 12853426
- PMCID: PMC6740347
- DOI: 10.1523/JNEUROSCI.23-14-06074.2003
Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse
Erratum in
- J Neurosci. 2004 Mar 3;24(9):2343
Abstract
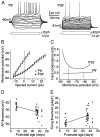
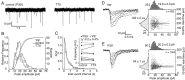
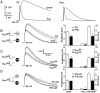
The timing of action potentials is an important determinant of information coding in the brain. The shape of the EPSP has a key influence on the temporal precision of spike generation. Here we use dynamic clamp recording and passive neuronal models to study how developmental changes in synaptic conductance waveform and intrinsic membrane properties combine to affect the EPSP and action potential generation in cerebellar granule cells. We recorded EPSCs at newly formed and mature mossy fiber-granule cell synapses. Both quantal and evoked currents showed a marked speeding of the AMPA receptor-mediated component. We also found evidence for age- and activity-dependent changes in the involvement of NMDA receptors. Although AMPA and NMDA receptors contributed to quantal EPSCs at immature synapses, multiquantal release was required to activate NMDA receptors at mature synapses, suggesting a developmental redistribution of NMDA receptors. These changes in the synaptic conductance waveform result in a faster rising EPSP and reduced spike latency in mature granule cells. Mature granule cells also have a significantly decreased input resistance, contributing to a faster decaying EPSP and a reduced spike jitter. We suggest that these concurrent developmental changes, which increase the temporal precision of EPSP-spike coupling, will increase the fidelity with which sensory information is processed within the input layer of the cerebellar cortex.
Figures






Similar articles
-
Context-dependent effects of NMDA receptors on precise timing information at the endbulb of Held in the cochlear nucleus.J Neurophysiol. 2009 Nov;102(5):2627-37. doi: 10.1152/jn.00111.2009. Epub 2009 Sep 2. J Neurophysiol. 2009. PMID: 19726731 Free PMC article.
-
Role of AMPA and NMDA receptors and back-propagating action potentials in spike timing-dependent plasticity.J Neurophysiol. 2010 Jan;103(1):47-54. doi: 10.1152/jn.00416.2009. Epub 2009 Oct 28. J Neurophysiol. 2010. PMID: 19864442
-
Light-evoked excitatory synaptic currents of X-type retinal ganglion cells.J Neurophysiol. 2000 Jun;83(6):3217-29. doi: 10.1152/jn.2000.83.6.3217. J Neurophysiol. 2000. PMID: 10848542
-
Sensory representations in cerebellar granule cells.Curr Opin Neurobiol. 2009 Aug;19(4):445-51. doi: 10.1016/j.conb.2009.07.003. Epub 2009 Aug 3. Curr Opin Neurobiol. 2009. PMID: 19651506 Review.
-
Data-driven modeling of synaptic transmission and integration.Prog Mol Biol Transl Sci. 2014;123:305-50. doi: 10.1016/B978-0-12-397897-4.00004-8. Prog Mol Biol Transl Sci. 2014. PMID: 24560150 Free PMC article. Review.
Cited by
-
Gene regulation via excitation and BDNF is mediated by induction and phosphorylation of the Etv1 transcription factor in cerebellar granule cells.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8734-9. doi: 10.1073/pnas.1206418109. Epub 2012 May 14. Proc Natl Acad Sci U S A. 2012. PMID: 22586091 Free PMC article.
-
Integration of asynchronously released quanta prolongs the postsynaptic spike window.J Neurosci. 2007 Jun 20;27(25):6684-91. doi: 10.1523/JNEUROSCI.0934-07.2007. J Neurosci. 2007. PMID: 17581955 Free PMC article.
-
Synaptic organization of the mouse cerebellar cortex in organotypic slice cultures.Cerebellum. 2006;5(4):243-56. doi: 10.1080/14734220600905317. Cerebellum. 2006. PMID: 17134987
-
Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex.J Neurosci. 2008 Sep 3;28(36):8955-67. doi: 10.1523/JNEUROSCI.5469-07.2008. J Neurosci. 2008. PMID: 18768689 Free PMC article.
-
Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage.PLoS One. 2013 May 31;8(5):e64828. doi: 10.1371/journal.pone.0064828. Print 2013. PLoS One. 2013. PMID: 23741401 Free PMC article.
References
-
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N ( 1994) Differential expression of five N-methyl-d-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol 347: 150–160. - PubMed
-
- Bekkers JM, Stevens CF ( 1989) NMDA and non-NMDA receptors are colocalized at individual excitatory synapses in cultured rat hippocampus. Nature 341: 230–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
