Caspase inhibitors promote vestibular hair cell survival and function after aminoglycoside treatment in vivo
- PMID: 12853430
- PMCID: PMC6740338
- DOI: 10.1523/JNEUROSCI.23-14-06111.2003
Caspase inhibitors promote vestibular hair cell survival and function after aminoglycoside treatment in vivo
Abstract
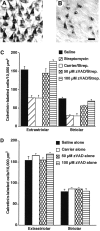
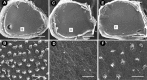
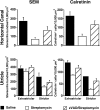
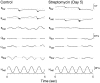
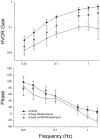
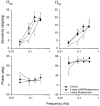
The sensory hair cells of the inner ear undergo apoptosis after acoustic trauma or aminoglycoside antibiotic treatment, causing permanent auditory and vestibular deficits in humans. Previous studies have demonstrated a role for caspase activation in hair cell death and ototoxic injury that can be reduced by concurrent treatment with caspase inhibitors in vitro. In this study, we examined the protective effects of caspase inhibition on hair cell death in vivo after systemic injections of aminoglycosides. In one series of experiments, chickens were implanted with osmotic pumps that administrated the pan-caspase inhibitor z-Val-Ala-Asp(Ome)-fluoromethylketone (zVAD) into inner ear fluids. One day after the surgery, the animals received a 5 d course of treatment with streptomycin, a vestibulotoxic aminoglycoside. Direct infusion of zVAD into the vestibule significantly increased hair cell survival after streptomycin treatment. A second series of experiments determined whether rescued hair cells could function as sensory receptors. Animals treated with streptomycin displayed vestibular system impairment as measured by a greatly reduced vestibulo-ocular response (VOR). In contrast, animals that received concurrent systemic administration of zVAD with streptomycin had both significantly greater hair cell survival and significantly increased VOR responses, as compared with animals treated with streptomycin alone. These findings suggest that inhibiting the activation of caspases promotes the survival of hair cells and protects against vestibular function deficits after aminoglycoside treatment.
Figures




Similar articles
-
Inhibition of caspases prevents ototoxic and ongoing hair cell death.J Neurosci. 2002 Feb 15;22(4):1218-27. doi: 10.1523/JNEUROSCI.22-04-01218.2002. J Neurosci. 2002. PMID: 11850449 Free PMC article.
-
Caspase activation in hair cells of the mouse utricle exposed to neomycin.J Neurosci. 2002 Oct 1;22(19):8532-40. doi: 10.1523/JNEUROSCI.22-19-08532.2002. J Neurosci. 2002. PMID: 12351727 Free PMC article.
-
Recovery of the vestibulocolic reflex after aminoglycoside ototoxicity in domestic chickens.J Neurophysiol. 1999 Mar;81(3):1025-35. doi: 10.1152/jn.1999.81.3.1025. J Neurophysiol. 1999. PMID: 10085330
-
Ongoing cell death and immune influences on regeneration in the vestibular sensory organs.Ann N Y Acad Sci. 2001 Oct;942:34-45. doi: 10.1111/j.1749-6632.2001.tb03733.x. Ann N Y Acad Sci. 2001. PMID: 11710476 Review.
-
Mechanisms of hair cell death and protection.Curr Opin Otolaryngol Head Neck Surg. 2005 Dec;13(6):343-8. doi: 10.1097/01.moo.0000186799.45377.63. Curr Opin Otolaryngol Head Neck Surg. 2005. PMID: 16282762 Review.
Cited by
-
Hsp70 inhibits aminoglycoside-induced hearing loss and cochlear hair cell death.Cell Stress Chaperones. 2009 Jul;14(4):427-37. doi: 10.1007/s12192-008-0097-2. Epub 2009 Jan 15. Cell Stress Chaperones. 2009. PMID: 19145477 Free PMC article.
-
Receptor-interacting protein kinases modulate noise-induced sensory hair cell death.Cell Death Dis. 2014 May 29;5(5):e1262. doi: 10.1038/cddis.2014.177. Cell Death Dis. 2014. PMID: 24874734 Free PMC article.
-
Molecular mechanisms of esophageal epithelial regeneration following repair of surgical defects with acellular silk fibroin grafts.Sci Rep. 2021 Mar 29;11(1):7086. doi: 10.1038/s41598-021-86511-9. Sci Rep. 2021. PMID: 33782465 Free PMC article.
-
Hepatocyte growth factor mimetic protects lateral line hair cells from aminoglycoside exposure.Front Cell Neurosci. 2015 Jan 28;9:3. doi: 10.3389/fncel.2015.00003. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25674052 Free PMC article.
-
Age-related hearing loss or presbycusis.Eur Arch Otorhinolaryngol. 2010 Aug;267(8):1179-91. doi: 10.1007/s00405-010-1270-7. Epub 2010 May 13. Eur Arch Otorhinolaryngol. 2010. PMID: 20464410 Review.
References
-
- Anastasio TJ, Correia MJ ( 1988) A frequency and time domain study of the horizontal and vertical vestibulo-ocular reflex in the pigeon. J Neurophysiol 59: 1143–1161. - PubMed
-
- Angelaki DE ( 1992) Two-dimensional coding of linear acceleration and the angular velocity sensitivity of the otolith system. Biol Cybern 67: 511–522. - PubMed
-
- Angelaki DE, Hess BJ ( 1996) Three-dimensional organization of otolithocular reflexes in rhesus monkeys. I. Linear acceleration responses during off-vertical axis rotation. J Neurophysiol 75: 2405–2424. - PubMed
-
- Aran JM, Chappert C, Dulon D, Erre JP, Aurousseau C ( 1995) Uptake of amikacin by hair cells of the guinea pig cochlea and vestibule and ototoxicity: comparison with gentamicin. Hear Res 82: 179–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
