GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina
- PMID: 12853434
- PMCID: PMC6740345
- DOI: 10.1523/JNEUROSCI.23-14-06152.2003
GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina
Abstract
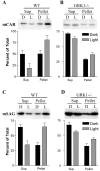
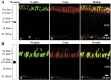
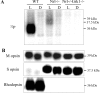
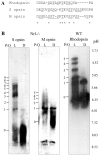
The shutoff mechanisms of the rod visual transduction cascade involve G-protein-coupled receptor (GPCR) kinase 1 (GRK1) phosphorylation of light-activated rhodopsin (R*) followed by rod arrestin binding. Deactivation of the cone phototransduction cascade in the mammalian retina is delineated poorly. In this study we sought to explore the potential mechanisms underlying the quenching of the phototransduction cascade in cone photoreceptors by using mouse models lacking rods and/or GRK1. Using the "pure-cone" retinas of the neural retina leucine zipper (Nrl) knock-out (KO, -/-) mice (Mears et al., 2001), we have demonstrated the light-dependent, multi-site phosphorylation of both S and M cone opsins by in situ phosphorylation and isoelectric focusing. Immunoprecipitation with affinity-purified polyclonal antibodies against either mouse cone arrestin (mCAR) or mouse S and M cone opsins revealed specific binding of mCAR to light-activated, phosphorylated cone opsins. To elucidate the potential role of GRK1 in cone opsin phosphorylation, we created Nrl and Grk1 double knock-out (Nrl-/-Grk1-/-) mice by crossing the Nrl-/- mice with Grk1-/- mice (Chen et al., 1999). We found that, in the retina of these mice, the light-activated cone opsins were neither phosphorylated nor bound with mCAR. Our results demonstrate, for the first time in a mammalian species, that cone opsins are phosphorylated and that CAR binds to phosphorylated cone opsins after light activation.
Figures




References
-
- Abdulaeva G, Hargrave PA, Smith WC ( 1995) The sequence of arrestins from rod and cone photoreceptors in the frogs Rana catesbeiana and Rana pipiens Localization of gene transcripts by reverse-transcription polymerase chain reaction on isolated photoreceptors. Eur J Biochem 234: 437–442. - PubMed
-
- Arshavsky V, Antoch MP, Philippov PP ( 1987) On the role of transducin GTPase in the quenching of a phosphodiesterase cascade of vision. FEBS Lett 224: 19–22. - PubMed
-
- Baylor DA, Burns ME ( 1998) Control of rhodopsin activity in vision. Eye 12: 521–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
