Diversity of protein-protein interactions
- PMID: 12853464
- PMCID: PMC165629
- DOI: 10.1093/emboj/cdg359
Diversity of protein-protein interactions
Abstract
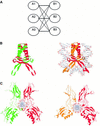
In this review, we discuss the structural and functional diversity of protein-protein interactions (PPIs) based primarily on protein families for which three-dimensional structural data are available. PPIs play diverse roles in biology and differ based on the composition, affinity and whether the association is permanent or transient. In vivo, the protomer's localization, concentration and local environment can affect the interaction between protomers and are vital to control the composition and oligomeric state of protein complexes. Since a change in quaternary state is often coupled with biological function or activity, transient PPIs are important biological regulators. Structural characteristics of different types of PPIs are discussed and related to their physiological function, specificity and evolution.
Figures



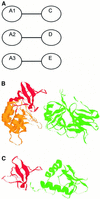
References
-
- Argos P. (1988) An investigation of protein subunit and domain interfaces. Protein Eng., 2, 101–113. - PubMed
-
- Brooijmans N., Sharp,K.A. and Kuntz,I.D. (2002) Stability of macromolecular complexes. Proteins, 48, 645–653. - PubMed
-
- Brown K., Nurizzo,D., Besson,S., Shepard,W., Moura,J., Moura,I., Tegoni,M. and Cambillau,C. (1999) MAD structure of Pseudomonas nautica dimeric cytochrome c552 mimicks the c4 Dihemic cytochrome domain association. J. Mol. Biol., 289, 1017–1028. - PubMed
-
- Chothia C. and Janin,J. (1975) Principles of protein–protein recognition. Nature, 256, 705–708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

