Mechanism of the electron transfer catalyst DsbB from Escherichia coli
- PMID: 12853466
- PMCID: PMC165626
- DOI: 10.1093/emboj/cdg356
Mechanism of the electron transfer catalyst DsbB from Escherichia coli
Abstract
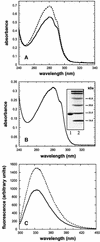
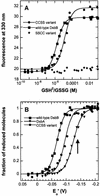
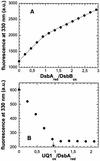
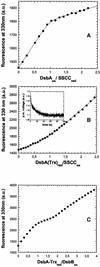
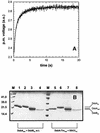
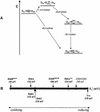
The membrane protein DsbB from Escherichia coli is essential for disulfide bond formation and catalyses the oxidation of the periplasmic dithiol oxidase DsbA by ubiquinone. DsbB contains two catalytic disulfide bonds, Cys41-Cys44 and Cys104-Cys130. We show that DsbB directly oxidizes one molar equivalent of DsbA in the absence of ubiquinone via disulfide exchange with the 104-130 disulfide bond, with a rate constant of 2.7 x 10 M(-1) x s(-1). This reaction occurs although the 104-130 disulfide is less oxidizing than the catalytic disulfide bond of DsbA (E(o)' = -186 and -122 mV, respectively). This is because the 41-44 disulfide, which is only accessible to ubiquinone but not to DsbA, is the most oxidizing disulfide bond in a protein described so far, with a redox potential of -69 mV. Rapid intramolecular disulfide exchange in partially reduced DsbB converts the enzyme into a state in which Cys41 and Cys44 are reduced and thus accessible for reoxidation by ubiquinone. This demonstrates that the high catalytic efficiency of DsbB results from the extreme intrinsic oxidative force of the enzyme.
Figures
References
-
- Bader M., Muse,W., Zander,T. and Bardwell,J. (1998) Reconstitution of a protein disulfide catalytic system. J. Biol. Chem., 273, 10302–10307. - PubMed
-
- Bader M., Muse,W., Ballou,D.P., Gassner,C. and Bardwell,J.C. (1999) Oxidative protein folding is driven by the electron transport system. Cell, 98, 217–227. - PubMed
-
- Bader M.W., Xie,T., Yu,C.A. and Bardwell,J.C. (2000) Disulfide bonds are generated by quinone reduction. J. Biol. Chem., 275, 26082–26088. - PubMed
-
- Bardwell J.C., McGovern,K. and Beckwith,J. (1991) Identification of a protein required for disulfide bond formation in vivo. Cell, 67, 581–589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

