T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema
- PMID: 12853470
- PMCID: PMC165612
- DOI: 10.1093/emboj/cdg342
T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema
Abstract
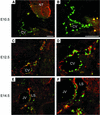
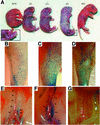
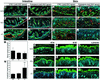
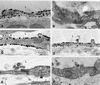
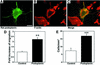
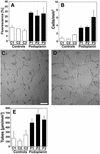
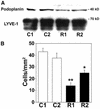
Within the vascular system, the mucin-type transmembrane glycoprotein T1alpha/podoplanin is predominantly expressed by lymphatic endothelium, and recent studies have shown that it is regulated by the lymphatic-specific homeobox gene Prox1. In this study, we examined the role of T1alpha/podoplanin in vascular development and the effects of gene disruption in mice. T1alpha/podoplanin is first expressed at around E11.0 in Prox1-positive lymphatic progenitor cells, with predominant localization in the luminal plasma membrane of lymphatic endothelial cells during later development. T1alpha/podoplanin(-/-) mice die at birth due to respiratory failure and have defects in lymphatic, but not blood vessel pattern formation. These defects are associated with diminished lymphatic transport, congenital lymphedema and dilation of lymphatic vessels. T1alpha/podoplanin is also expressed in the basal epidermis of newborn wild-type mice, but gene disruption did not alter epidermal differentiation. Studies in cultured endothelial cells indicate that T1alpha/podoplanin promotes cell adhesion, migration and tube formation, whereas small interfering RNA-mediated inhibition of T1alpha/podoplanin expression decreased lymphatic endothelial cell adhesion. These data identify T1alpha/podoplanin as a novel critical player that regulates different key aspects of lymphatic vasculature formation.
Figures


References
-
- Bartos V., Kolc,J., Vanecek,R. and Malek,P. (1979) Lymph and blood enzymes and pathologic alterations in canine experimental pancreatitis after administration of benzo-pyrones. Scand. J. Gastroenterol., 14, 343–347. - PubMed
-
- Carmeliet P. (2000) Mechanisms of angiogenesis and arteriogenesis. Nat. Med., 6, 389–395. - PubMed
-
- Dobbs L.G., Williams,M.C. and Gonzalez,R. (1988) Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim. Biophys Acta, 970, 146–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

