Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway
- PMID: 12853473
- PMCID: PMC165613
- DOI: 10.1093/emboj/cdg343
Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway
Abstract
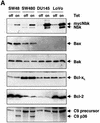
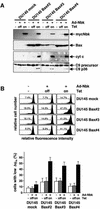
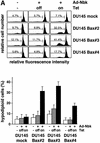
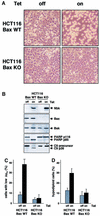
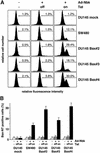
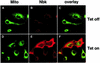
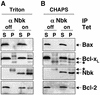
Nbk/Bik (natural born killer/Bcl-2-interacting killer) is a tissue-specific BH3-only protein whose molecular function is still largely unknown. To investigate the mechanism of Nbk action, we established a single- vector adenoviral system based on the Tet-off conditional expression of Nbk. Upon Nbk expression, only Bax-positive, but not Bax-deficient cells were found to undergo apoptosis. Interestingly, Nbk failed to induce apoptosis in the absence of Bax, even despite expression of the related molecule Bak. Re-expression of Bax restored the sensitivity to Nbk. Similarly, Bax wild-type HCT116 cells were highly susceptible, whereas HCT116 Bax knock-out cells remained resistant to Nbk-induced apoptosis. In Bax-positive cells, Nbk induced a conformational switch in the Bax N-terminus coinciding with cytochrome c release, mitochondrial permeability transition and caspase-9 processing. Immunoprecipitation studies revealed that Nbk interacts with Bcl-x(L) and Bcl-2 but not with Bax. Since, in addition, Nbk did not localize to the mitochondria, our data suggest a model in which Nbk acts as an indirect killer to trigger Bax-dependent apoptosis, whereas Bak is not sufficient to confer sensitivity to Nbk.
Figures





References
-
- Borner C. (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol. Immunol., 39, 615–647. - PubMed
-
- Boyd J.M. et al. (1995) Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene, 11, 1921–1928. - PubMed
-
- Cheng E.H., Wei,M.C., Weiler,S., Flavell,R.A., Mak,T.W., Lindsten,T. and Korsmeyer,S.J. (2001) Bcl-2, Bcl-xL sequester BH3 domain-only molecules preventing Bax- and Bak-mediated mitochondrial apoptosis. Mol. Cell, 8, 705–711. - PubMed
-
- Daniel P.T., Pun,K.T., Ritschel,S., Sturm,I., Holler,J., Dörken,B. and Brown,R. (1999) Expression of the death gene Bik/Nbk promotes sensitivity to drug-induced apoptosis in corticosteroid-resistant T-cell lymphoma and prevents tumor growth in severe combined immunodeficient mice. Blood, 94, 1100–1107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

