Cofactor Tpr2 combines two TPR domains and a J domain to regulate the Hsp70/Hsp90 chaperone system
- PMID: 12853476
- PMCID: PMC165632
- DOI: 10.1093/emboj/cdg362
Cofactor Tpr2 combines two TPR domains and a J domain to regulate the Hsp70/Hsp90 chaperone system
Abstract
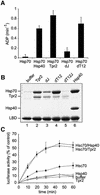
In the eukaryotic cytosol, Hsp70 and Hsp90 cooperate with various co-chaperone proteins in the folding of a growing set of substrates, including the glucocorticoid receptor (GR). Here, we analyse the function of the co-chaperone Tpr2, which contains two chaperone-binding TPR domains and a DnaJ homologous J domain. In vivo, an increase or decrease in Tpr2 expression reduces GR activation, suggesting that Tpr2 is required at a narrowly defined expression level. As shown in vitro, Tpr2 recognizes both Hsp70 and Hsp90 through its TPR domains, and its J domain stimulates ATP hydrolysis and polypeptide binding by Hsp70. Furthermore, unlike other co-chaperones, Tpr2 induces ATP-independent dissociation of Hsp90 but not of Hsp70 from chaperone-substrate complexes. Excess Tpr2 inhibits the Hsp90-dependent folding of GR in cell lysates. We propose a novel mechanism in which Tpr2 mediates the retrograde transfer of substrates from Hsp90 onto Hsp70. At normal levels substoichiometric to Hsp90 and Hsp70, this activity optimizes the function of the multichaperone machinery.
Figures






References
-
- Brinker A., Scheufler,C., Von Der Mulbe,F., Fleckenstein,B., Herrmann,C., Jung,G., Moarefi,I. and Hartl,F.U. (2002) Ligand discrimination by TPR domain. Relevance and selectivity of EEVD-recognition in Hsp70–Hop–Hsp90 complexes. J. Biol. Chem., 277, 19265–19275. - PubMed
-
- Buchner J. (1999) Hsp90 & Co.—a holding for folding. Trends Biochem. Sci., 24, 136–141. - PubMed
-
- Bukau B. and Horwich,A. (1998) The Hsp70 and Hsp60 chaperone machines. Cell, 92, 351–366. - PubMed
-
- Chen S. and Smith,D.F. (1998) Hop as an adaptor in the heat shock protein 70 (Hsp70) and Hsp90 chaperone machinery. J. Biol. Chem., 273, 35194–35200. - PubMed
-
- Dittmar K.D., Demady,D.R., Stancato,L.F., Krishna,P. and Pratt,W.B. (1997) Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor–hsp90 heterocomplexes formed by hsp90–p60–hsp70. J. Biol. Chem., 272, 21213–21220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

