Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure
- PMID: 12853572
- PMCID: PMC166433
- DOI: 10.1073/pnas.1530287100
Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure
Abstract
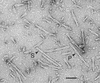
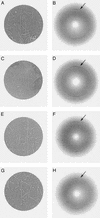
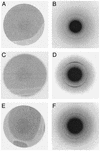
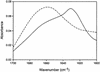
Abnormal filaments consisting of hyperphosphorylated microtubule-associated protein tau form in the brains of patients with Alzheimer's disease, Down's syndrome, and various dementing tauopathies. In Alzheimer's disease and Down's syndrome, the filaments have two characteristic morphologies referred to as paired helical and straight filaments, whereas in tauopathies, there is a wider range of morphologies. There has been controversy in the literature concerning the internal molecular fine structure of these filaments, with arguments for and against the cross-beta structure demonstrated in many other amyloid fibers. The difficulty is to produce from brain pure preparations of filaments for analysis. One approach to avoid the need for a pure preparation is to use selected area electron diffraction from small groups of filaments of defined morphology. Alternatively, it is possible to assemble filaments in vitro from expressed tau protein to produce a homogeneous specimen suitable for analysis by electron diffraction, x-ray diffraction, and Fourier transform infrared spectroscopy. Using both these approaches, we show here that native filaments from brain and filaments assembled in vitro from expressed tau protein have a clear cross-beta structure.
Figures

References
-
- St George-Hyslop, P. H., Farrer, L. A. & Goedert, M. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (McGraw–Hill, New York), pp. 5875-5899.
-
- Crowther, R. A. & Goedert, M. (2000) J. Struct. Biol. 130, 271-279. - PubMed
-
- Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. (1989) Neuron 3, 519-526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

