Anti-nerve growth factor Ab abrogates macrophage-mediated HIV-1 infection and depletion of CD4+ T lymphocytes in hu-SCID mice
- PMID: 12853577
- PMCID: PMC166415
- DOI: 10.1073/pnas.1332627100
Anti-nerve growth factor Ab abrogates macrophage-mediated HIV-1 infection and depletion of CD4+ T lymphocytes in hu-SCID mice
Abstract
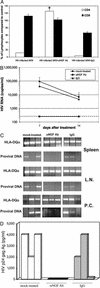
Infection by HIV-1 causes persistent, long-term high virus production in macrophages. Major evidence, both in humans and in primate models, shows the crucial role of macrophages in sustaining virus production and in mediating a cytopathic effect on bystander CD4+ T lymphocytes and neuronal cells. In the present study, we used severe combined immunodeficient (SCID) mice engrafted with human peripheral blood lymphocytes (hu-PBL-SCID mice) to investigate the in vivo effect of HIV-1-infected macrophages on virus spread and CD4+ T lymphocyte depletion, and the ability of a mAb against nerve growth factor (NGF, a neurokine essential for the survival of HIV-1-infected macrophages) to suppress the pathogenetic events mediated by infected macrophages. Injection of mice with as few as 500 HIV-exposed macrophages causes (i) complete depletion of several millions of autologous CD4+ T lymphocytes, (ii) sustained HIV viremia, and (iii) spreading of HIV-1 DNA in mouse lymphoid organs. In contrast, in vivo treatment with an anti-NGF Ab completely abrogates all effects mediated by HIV-infected macrophages. Taken together, the results demonstrate the remarkable power of macrophages in sustaining in vivo HIV-1 infection, and that such a phenomenon can be specifically abrogated by an anti-NGF Ab. This may open new perspectives of experimental approaches aimed at selectively eliminating persistently infected macrophages from the bodies of HIV-infected patients.
Figures



Similar articles
-
The human HIV/peripheral blood lymphocyte (PBL)-SCID mouse. A modified human PBL-SCID model for the study of HIV pathogenesis and therapy.J Immunol. 1995 Jun 15;154(12):6612-23. J Immunol. 1995. PMID: 7759895
-
Type I interferon is a powerful inhibitor of in vivo HIV-1 infection and preserves human CD4(+) T cells from virus-induced depletion in SCID mice transplanted with human cells.Virology. 1999 Oct 10;263(1):78-88. doi: 10.1006/viro.1999.9869. Virology. 1999. PMID: 10544084
-
Identification of HIV-1 epitopes that induce the synthesis of a R5 HIV-1 suppression factor by human CD4+ T cells isolated from HIV-1 immunized hu-PBL SCID mice.Clin Dev Immunol. 2005 Dec;12(4):235-42. doi: 10.1080/17402520500391557. Clin Dev Immunol. 2005. PMID: 16584108 Free PMC article.
-
Macrophages: a crucial reservoir for human immunodeficiency virus in the body.J Biol Regul Homeost Agents. 2001 Jul-Sep;15(3):272-6. J Biol Regul Homeost Agents. 2001. PMID: 11693436 Review.
-
SCID-hu mice: a model for studying disseminated HIV infection.Semin Immunol. 1996 Aug;8(4):223-31. doi: 10.1006/smim.1996.0028. Semin Immunol. 1996. PMID: 8883145 Review.
Cited by
-
The contribution of peroxynitrite generation in HIV replication in human primary macrophages.Retrovirology. 2007 Oct 21;4:76. doi: 10.1186/1742-4690-4-76. Retrovirology. 2007. PMID: 17949509 Free PMC article.
-
Nerve growth factor stimulation promotes CXCL-12 attraction of monocytes but decreases human immunodeficiency virus replication in attracted population.J Neurovirol. 2009 Jan;15(1):71-80. doi: 10.1080/13550280802482575. Epub 2008 Nov 19. J Neurovirol. 2009. PMID: 19023688
-
Single cycle structure-based humanization of an anti-nerve growth factor therapeutic antibody.PLoS One. 2012;7(3):e32212. doi: 10.1371/journal.pone.0032212. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403636 Free PMC article.
-
The engagement of activating FcgammaRs inhibits primate lentivirus replication in human macrophages.J Immunol. 2006 Nov 1;177(9):6291-300. doi: 10.4049/jimmunol.177.9.6291. J Immunol. 2006. PMID: 17056559 Free PMC article.
-
Potent anti-R5 human immunodeficiency virus type 1 effects of a CCR5 antagonist, AK602/ONO4128/GW873140, in a novel human peripheral blood mononuclear cell nonobese diabetic-SCID, interleukin-2 receptor gamma-chain-knocked-out AIDS mouse model.J Virol. 2005 Feb;79(4):2087-96. doi: 10.1128/JVI.79.4.2087-2096.2005. J Virol. 2005. PMID: 15681411 Free PMC article.
References
-
- Chun, T.-W., Davey, R. T., Ostrowski, M., Shawn Justement, J., Engel, D., Mullins, J. I. & Fauci, A. S. (2000) Nat. Med. 6, 757–761. - PubMed
-
- Lambotte, O., Taoufik, Y., de Goer, M. G., Wallon, C., Goujard, C. & Delfraissy, J. F. (2000) J. Acquired Immune Defic. Syndr. 23, 114–119. - PubMed
-
- Herbein, G., Coaquette, A., Perez-Bercoff, D. & Pancino, G. (2002) Curr. Mol. Med. 2, 723–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

