Temperature-sensitive mutation in yeast mitochondrial ribosome recycling factor (RRF)
- PMID: 12853640
- PMCID: PMC165964
- DOI: 10.1093/nar/gkg449
Temperature-sensitive mutation in yeast mitochondrial ribosome recycling factor (RRF)
Abstract
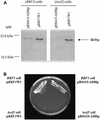
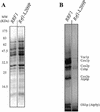
The yeast protein Rrf1p encoded by the FIL1 nuclear gene bears significant sequence similarity to Escherichia coli ribosome recycling factor (RRF). Here, we call FIL1 Ribosome Recycling Factor of yeast, RRF1. Its gene product, Rrf1p, was localized in mitochondria. Deletion of RRF1 leads to a respiratory incompetent phenotype and to instability of the mitochondrial genome (conversion to rho(-)/rho(0) cytoplasmic petites). Yeast with intact mitochondria and with deleted genomic RRF1 that harbors a plasmid carrying RRF1 was prepared from spores of heterozygous diploid yeast. Such yeast with a mutated allele of RRF1, rrf1-L209P, grew on a non-fermentable carbon source at 30 but not at 36 degrees C, where mitochondrial but not total protein synthesis was 90% inhibited. We propose that Rrf1p is essential for mitochondrial protein synthesis and acts as a RRF in mitochondria.
Figures




Similar articles
-
Ribosome recycling defects modify the balance between the synthesis and assembly of specific subunits of the oxidative phosphorylation complexes in yeast mitochondria.Nucleic Acids Res. 2016 Jul 8;44(12):5785-97. doi: 10.1093/nar/gkw490. Epub 2016 Jun 1. Nucleic Acids Res. 2016. PMID: 27257059 Free PMC article.
-
A regulatory factor, Fil1p, involved in derepression of the isocitrate lyase gene in Saccharomyces cerevisiae--a possible mitochondrial protein necessary for protein synthesis in mitochondria.Eur J Biochem. 1998 Aug 15;256(1):212-20. doi: 10.1046/j.1432-1327.1998.2560212.x. Eur J Biochem. 1998. PMID: 9746366
-
Chemicals or mutations that target mitochondrial translation can rescue the respiratory deficiency of yeast bcs1 mutants.Biochim Biophys Acta Mol Cell Res. 2017 Dec;1864(12):2297-2307. doi: 10.1016/j.bbamcr.2017.09.003. Epub 2017 Sep 6. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28888990
-
Organization and dynamics of yeast mitochondrial nucleoids.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(5):339-359. doi: 10.2183/pjab.93.021. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28496055 Free PMC article. Review.
-
Mitochondrial inheritance in yeast.Biochim Biophys Acta. 2014 Jul;1837(7):1039-46. doi: 10.1016/j.bbabio.2013.10.005. Epub 2013 Oct 29. Biochim Biophys Acta. 2014. PMID: 24183694 Review.
Cited by
-
Broad metabolic sensitivity profiling of a prototrophic yeast deletion collection.Genome Biol. 2014 Apr 10;15(4):R64. doi: 10.1186/gb-2014-15-4-r64. Genome Biol. 2014. PMID: 24721214 Free PMC article.
-
Mechanisms of ribosome recycling in bacteria and mitochondria: a structural perspective.RNA Biol. 2022;19(1):662-677. doi: 10.1080/15476286.2022.2067712. Epub 2021 Dec 31. RNA Biol. 2022. PMID: 35485608 Free PMC article. Review.
-
Mitochondrial ribosomes in cancer.Semin Cancer Biol. 2017 Dec;47:67-81. doi: 10.1016/j.semcancer.2017.04.004. Epub 2017 Apr 23. Semin Cancer Biol. 2017. PMID: 28445780 Free PMC article. Review.
-
Ribosome recycling step in yeast cytoplasmic protein synthesis is catalyzed by eEF3 and ATP.Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):10854-9. doi: 10.1073/pnas.1006247107. Epub 2010 Jun 1. Proc Natl Acad Sci U S A. 2010. PMID: 20534490 Free PMC article.
-
Triacylglycerol mobilization underpins mitochondrial stress recovery.Nat Cell Biol. 2025 Feb;27(2):298-308. doi: 10.1038/s41556-024-01586-6. Epub 2025 Jan 8. Nat Cell Biol. 2025. PMID: 39779944 Free PMC article.
References
-
- Suzuki T., Terasaki,M., Takemoto-Hori,C., Hanada,T., Ueda,T., Wada,A. and Watanabe,K. (2001) Proteomic analysis of the mammalian mitochondrial ribosome. Identification of protein components in the 28 S small subunit. J. Biol. Chem., 11, 33181–33195. - PubMed
-
- O’Brien T.W., Liu,J., Sylvester,J.E., Mougey,E.B., Fischel-Ghodsian,N., Thiede,B., Wittmann-Liebold,B. and Graack,H.R. (2000) Mammalian mitochondrial ribosomal proteins (4). Amino acid sequencing, characterization and identification of corresponding gene sequences. J. Biol. Chem., 275, 18153–18159. - PubMed
-
- Daum G., Bohni,P.C. and Schatz,G. (1982) Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem., 257, 13028–13033. - PubMed
-
- Landrieu I., Vandenbol,M., Hartlein,M. and Portetelle,D. (1997) Mitochondrial asparaginyl-tRNA synthetase is encoded by the yeast nuclear gene YCR24c. Eur. J. Biochem., 243, 268–273. - PubMed
-
- Fox T.D. (1996) Genetics of mitochondrial translation. In Hershey,J.W.B., Mathews,M.B. and Sonnenberg,N. (eds), Translational Control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

