A new Thermus sp. class-IIS enzyme sub-family: isolation of a 'twin' endonuclease TspDTI with a novel specificity 5'-ATGAA(N(11/9))-3', related to TspGWI, TaqII and Tth111II
- PMID: 12853651
- PMCID: PMC167652
- DOI: 10.1093/nar/gng074
A new Thermus sp. class-IIS enzyme sub-family: isolation of a 'twin' endonuclease TspDTI with a novel specificity 5'-ATGAA(N(11/9))-3', related to TspGWI, TaqII and Tth111II
Abstract
The TspDTI restriction endonuclease, which shows a novel recognition specificity 5'-ATGAA(N(11/9))-3', was isolated from Thermus sp. DT. TspDTI appears to be a 'twin' of restriction endonuclease TspGWI from Thermus sp. GW, as we have previously reported. TspGWI was isolated from the same location as TspDTI, it recognizes a related sequence 5'-ACGGA(N(11/9))-3' and has conserved cleavage positions. Both enzymes resemble two other class-IIS endonucleases from Thermus sp.: TaqII and Tth111II. N-terminal amino acid sequences of TspGWI tryptic peptides exhibit 88.9-100% similarity to the TaqII sequence. All four enzymes were purified to homogeneity; their polypeptide sizes (114.5-122 kDa) make them the largest class-IIS restriction endonucleases known to date. The existence of a Thermus sp. sub-family of class-IIS restriction endonucleases of a common origin is herein proposed.
Figures


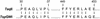
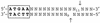
Similar articles
-
Bifunctional TaqII restriction endonuclease: redefining the prototype DNA recognition site and establishing the Fidelity Index for partial cleaving.BMC Biochem. 2011 Dec 5;12:62. doi: 10.1186/1471-2091-12-62. BMC Biochem. 2011. PMID: 22141927 Free PMC article.
-
Related bifunctional restriction endonuclease-methyltransferase triplets: TspDTI, Tth111II/TthHB27I and TsoI with distinct specificities.BMC Mol Biol. 2012 Apr 10;13:13. doi: 10.1186/1471-2199-13-13. BMC Mol Biol. 2012. PMID: 22489904 Free PMC article.
-
TspGWI, a thermophilic class-IIS restriction endonuclease from Thermus sp., recognizes novel asymmetric sequence 5'-ACGGA(N11/9)-3'.Nucleic Acids Res. 2002 Apr 1;30(7):e33. doi: 10.1093/nar/30.7.e33. Nucleic Acids Res. 2002. PMID: 11917039 Free PMC article.
-
Cloning and analysis of a bifunctional methyltransferase/restriction endonuclease TspGWI, the prototype of a Thermus sp. enzyme family.BMC Mol Biol. 2009 May 29;10:52. doi: 10.1186/1471-2199-10-52. BMC Mol Biol. 2009. PMID: 19480701 Free PMC article.
-
Class-IIS restriction enzymes--a review.Gene. 1991 Apr;100:13-26. doi: 10.1016/0378-1119(91)90345-c. Gene. 1991. PMID: 2055464 Review.
Cited by
-
Characterization of cleavage intermediate and star sites of RM.Tth111II.Sci Rep. 2014 Jan 23;4:3838. doi: 10.1038/srep03838. Sci Rep. 2014. PMID: 24452415 Free PMC article.
-
The MmeI family: type II restriction-modification enzymes that employ single-strand modification for host protection.Nucleic Acids Res. 2009 Aug;37(15):5208-21. doi: 10.1093/nar/gkp534. Epub 2009 Jul 3. Nucleic Acids Res. 2009. PMID: 19578066 Free PMC article.
-
Cofactor analogue-induced chemical reactivation of endonuclease activity in a DNA cleavage/methylation deficient TspGWI N₄₇₃A variant in the NPPY motif.Mol Biol Rep. 2014;41(4):2313-23. doi: 10.1007/s11033-014-3085-x. Epub 2014 Jan 19. Mol Biol Rep. 2014. PMID: 24442320 Free PMC article.
-
Modified 'one amino acid-one codon' engineering of high GC content TaqII-coding gene from thermophilic Thermus aquaticus results in radical expression increase.Microb Cell Fact. 2014 Jan 11;13:7. doi: 10.1186/1475-2859-13-7. Microb Cell Fact. 2014. PMID: 24410856 Free PMC article.
-
Novel parameter describing restriction endonucleases: Secondary-Cognate-Specificity and chemical stimulation of TsoI leading to substrate specificity change.Appl Microbiol Biotechnol. 2019 Apr;103(8):3439-3451. doi: 10.1007/s00253-019-09731-0. Epub 2019 Mar 16. Appl Microbiol Biotechnol. 2019. PMID: 30879089 Free PMC article.
References
-
- Roberts R.J. and Halford,S.E. (1993) Type II restriction endonucleases. In Linn,S.M., Lloyd,R.S. and Roberts,R.J. (eds), Nucleases, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 35–88.
-
- Endlish B. and Linn,S. (1985) The DNA restriction endonuclease of Escherichia coli B. I, II. J. Biol. Chem., 260, 5720–5738. - PubMed
-
- Bickle T.A. (1993) The ATP-dependent restriction enzymes. In Linn,S.M., Lloyd,R.S. and Roberts,R.J. (eds), Nucleases, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 89–109.
-
- Szybalski W., Kim,S.C., Hasan,N. and Podhajska,A.J. (1991) Class-IIS restriction enzymes—a review. Gene, 100, 13–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

